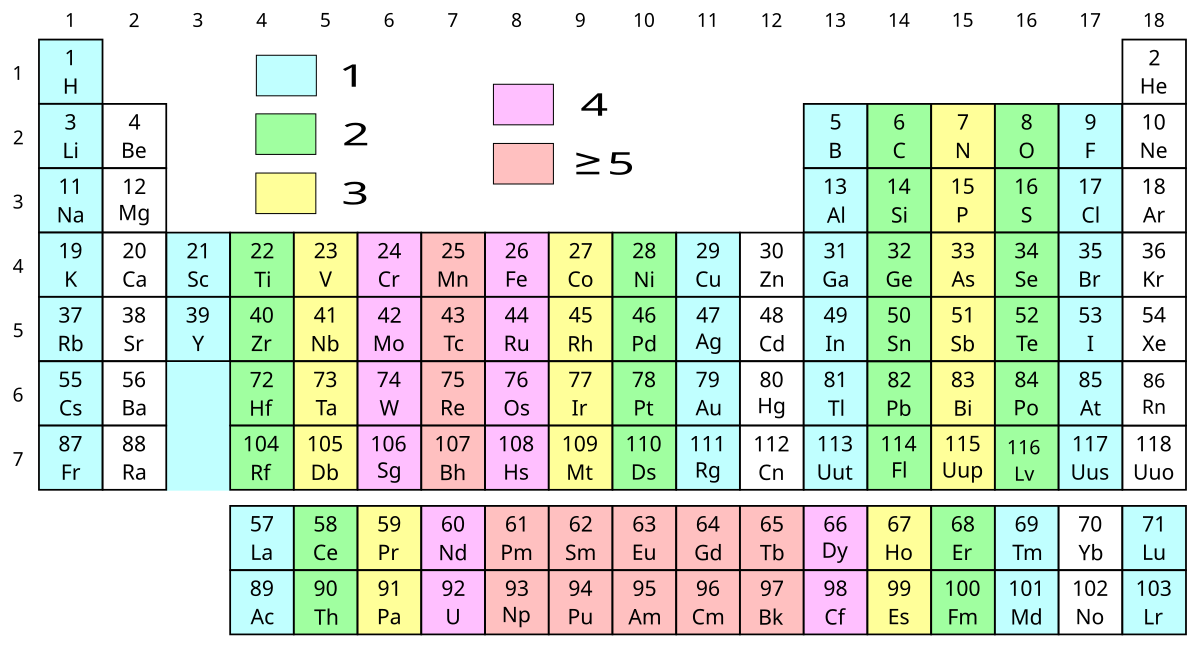
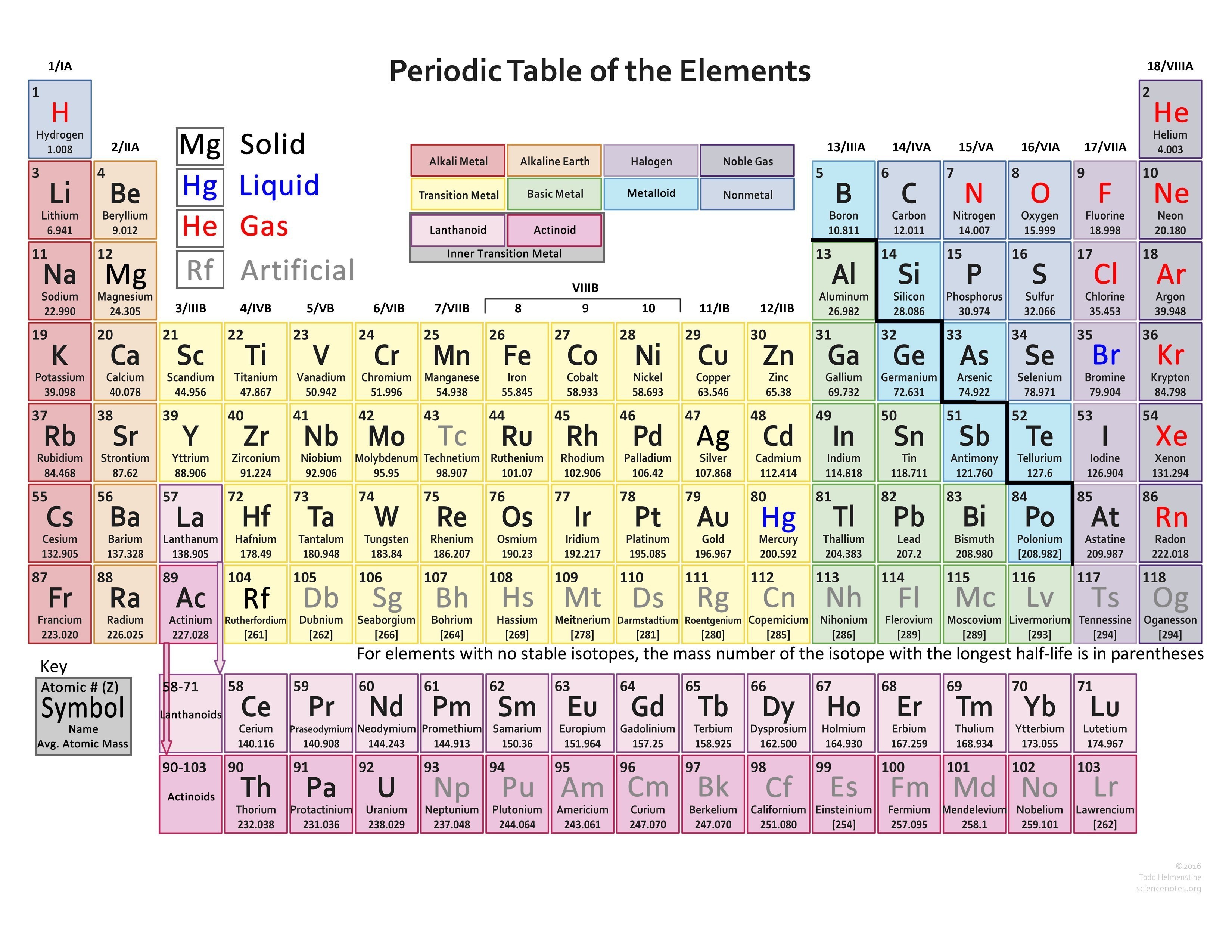
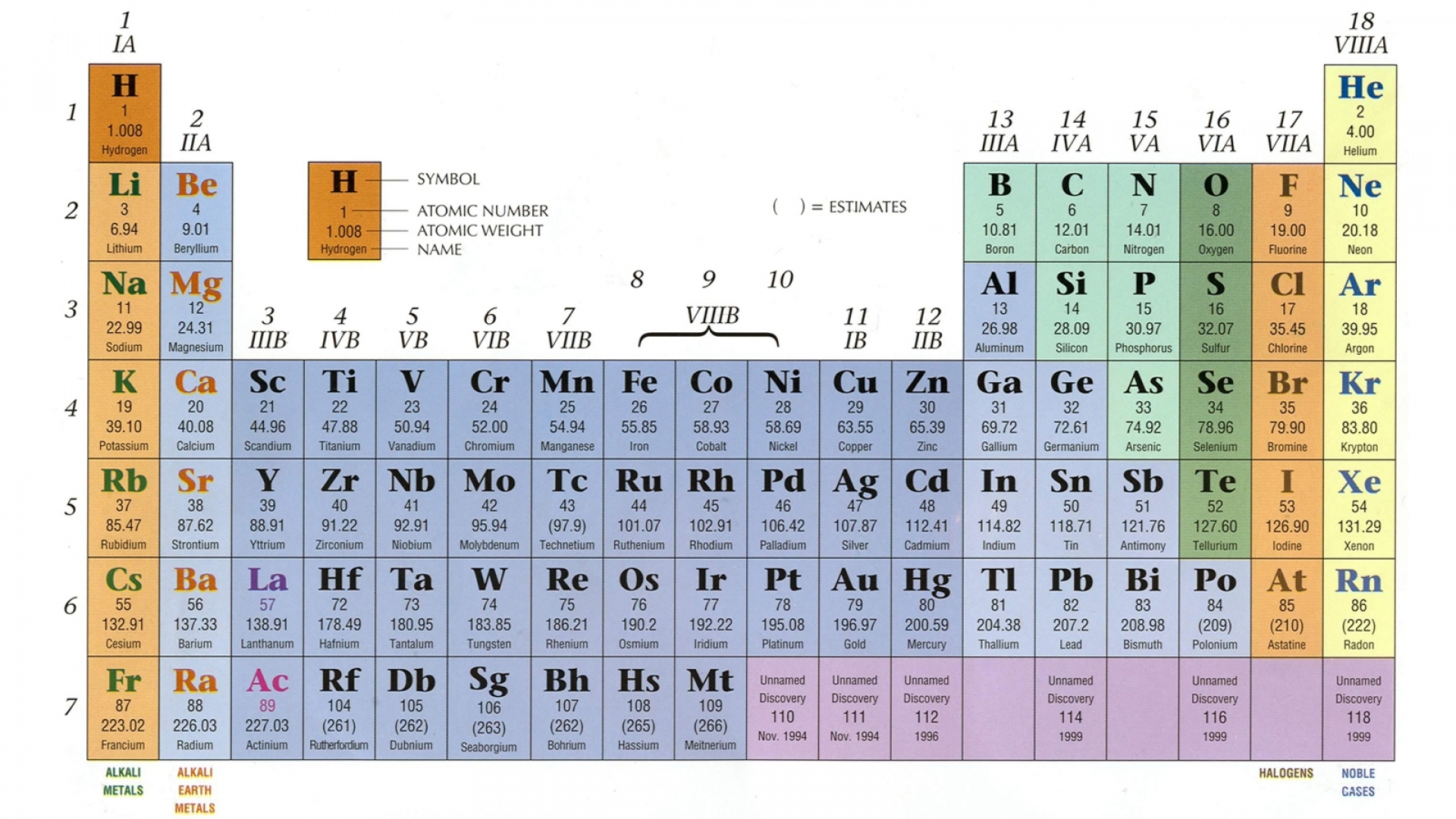
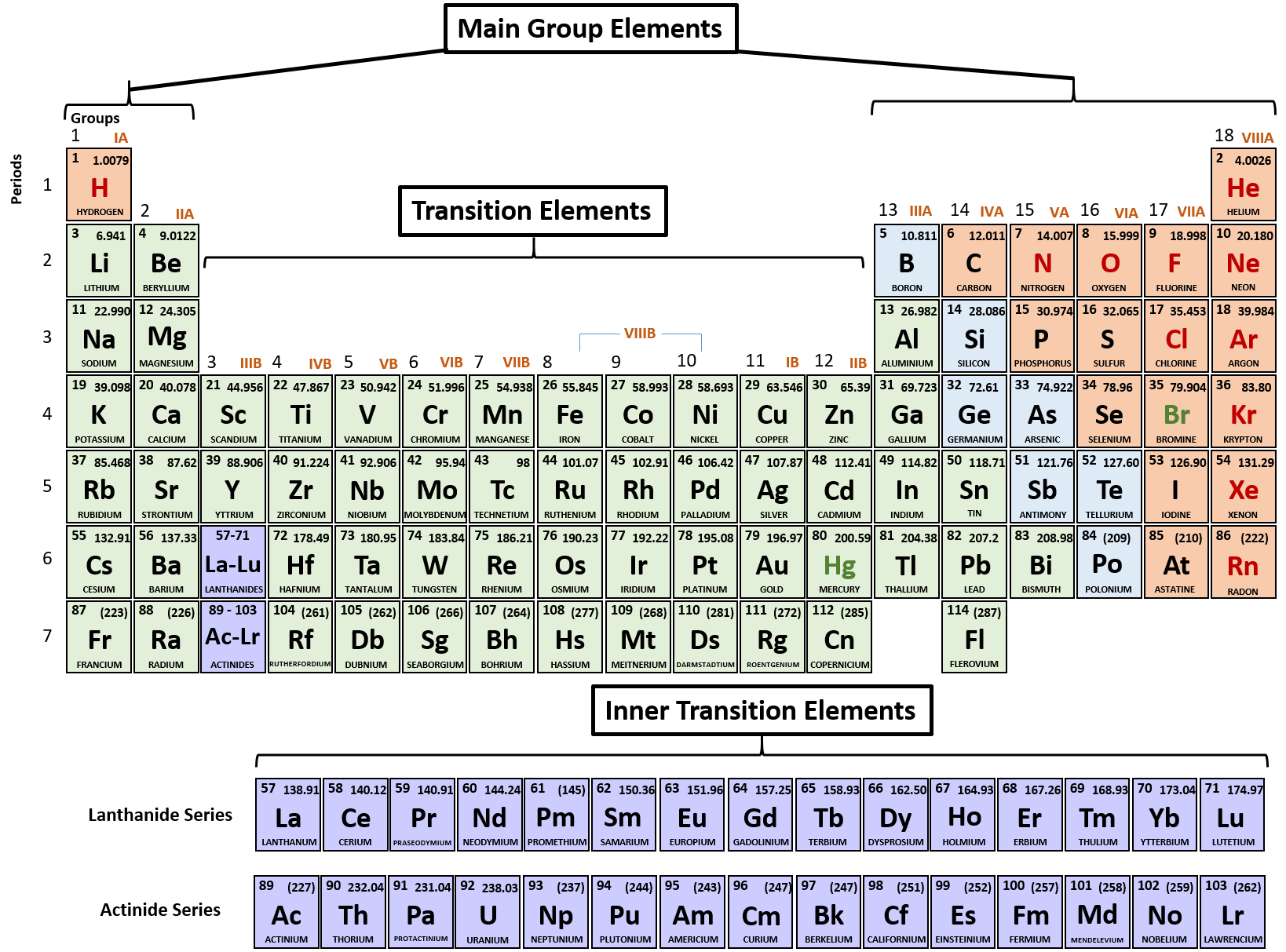
Periodic Table Valence Electrons Chart
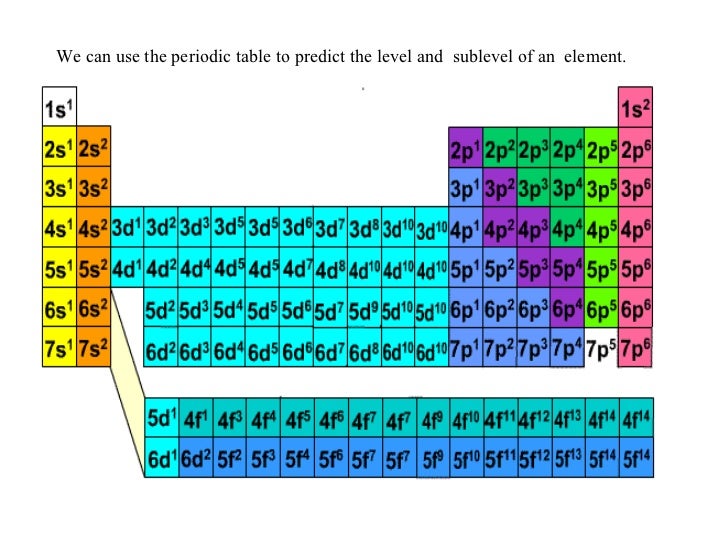
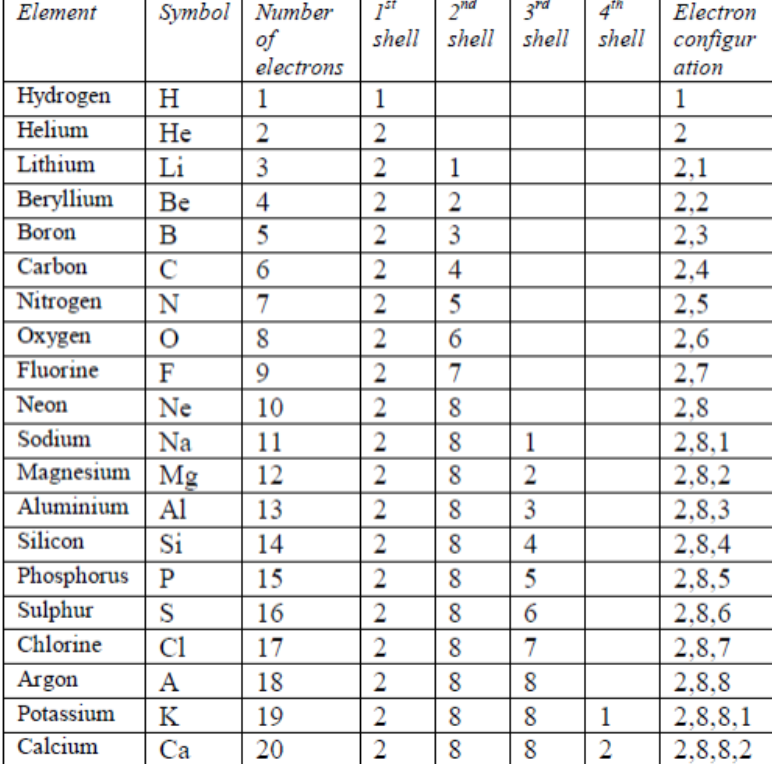
Each element has a unique atomic structure that is influenced by its electronic configuration which is the distribution of electrons across different orbitals of an atom. While these are the most common valences the real behavior of electrons is less simple.
This valence electrons chart table gives the valence electrons of all the elements of periodic table.

Periodic table valence electrons chart. This article provides you with an electronic configuration chart for all these elements. One of the easiest ways to find valence electrons is by checking out the elements place in the periodic table. A simpler version listing only the most common valence charges is also available.
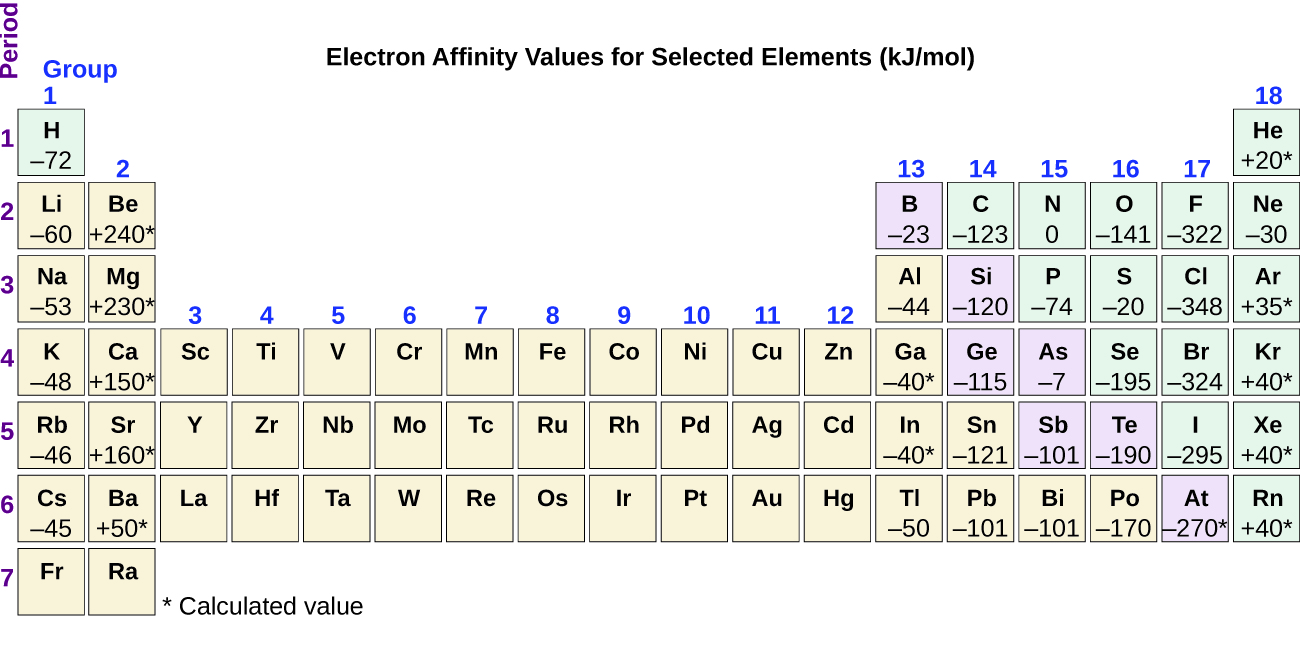
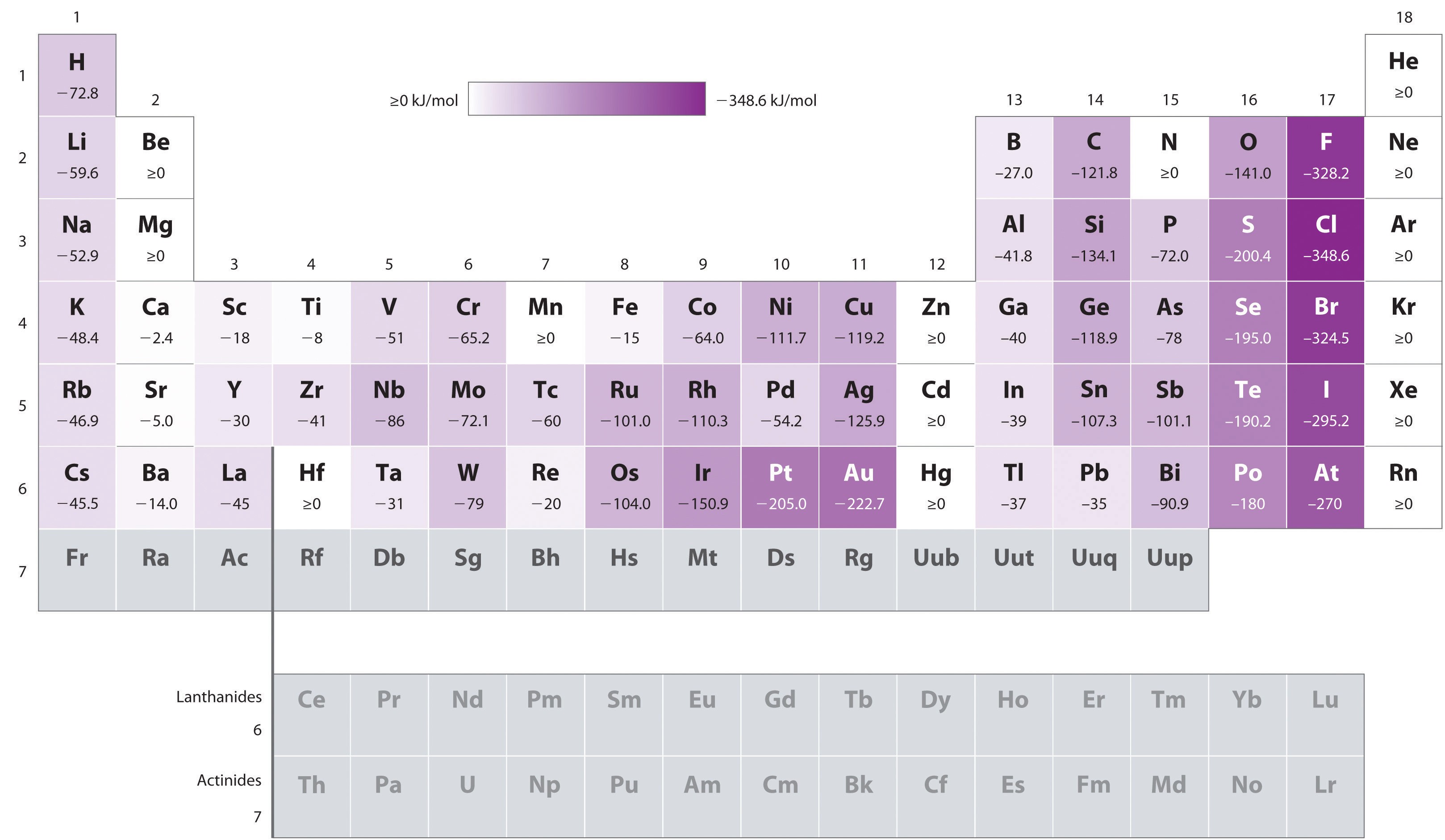
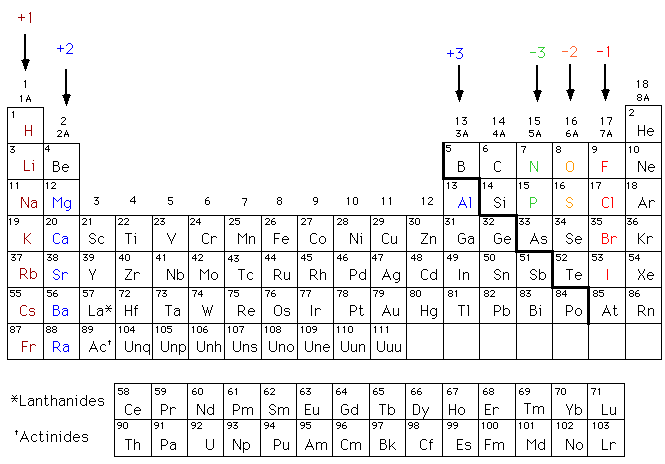
This is a table of the valences or oxidation states of the elements. The most common valences are in bold. Click here to buy a book photographic periodic table poster card deck or 3d print based on the images you see here.
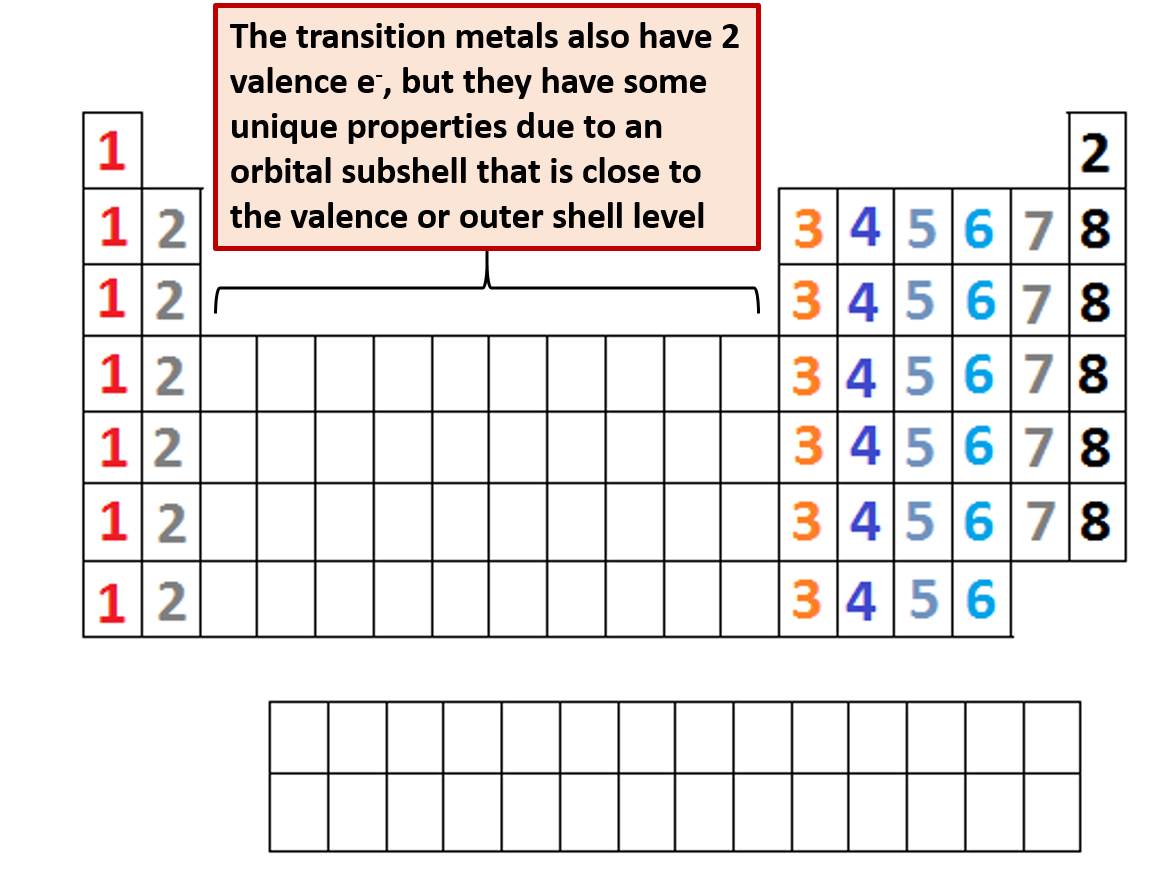
For example atoms in groups 1 and 2 have 1 and 2 valence electrons respectively. This information is available on a color periodic table of the elements or a black and white version. You may assume that the valences of the elementsthe number of electrons with which an atom will bond or formare those that can be derived by looking at the groups columns of the periodic table.
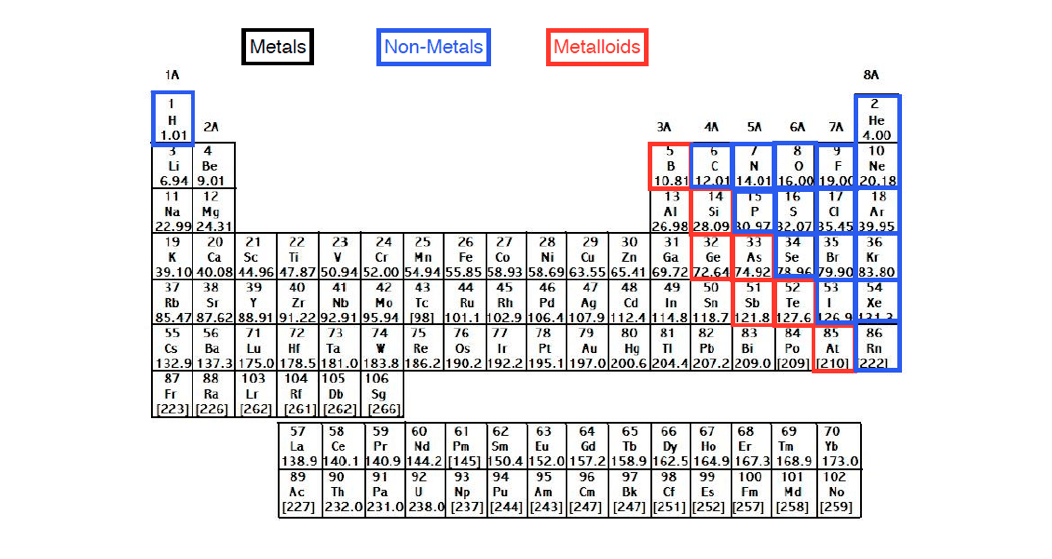
The valence electrons of an element can be found out by closely examining the vertical column in which the elements are grouped in. These elements are known as inner transition metals. Knowing how to find the number of valence electrons in a particular atom is an important skill for chemists because this information determines the kinds of chemical bonds that it can form and therefore the elements reactivity.
There are 118 elements in the periodic table. You can easily determine the number of valence electrons an atom can have by looking at its group in the periodic table. Luckily all you need to find an elements valence electrons is a standard periodic table of the elements.
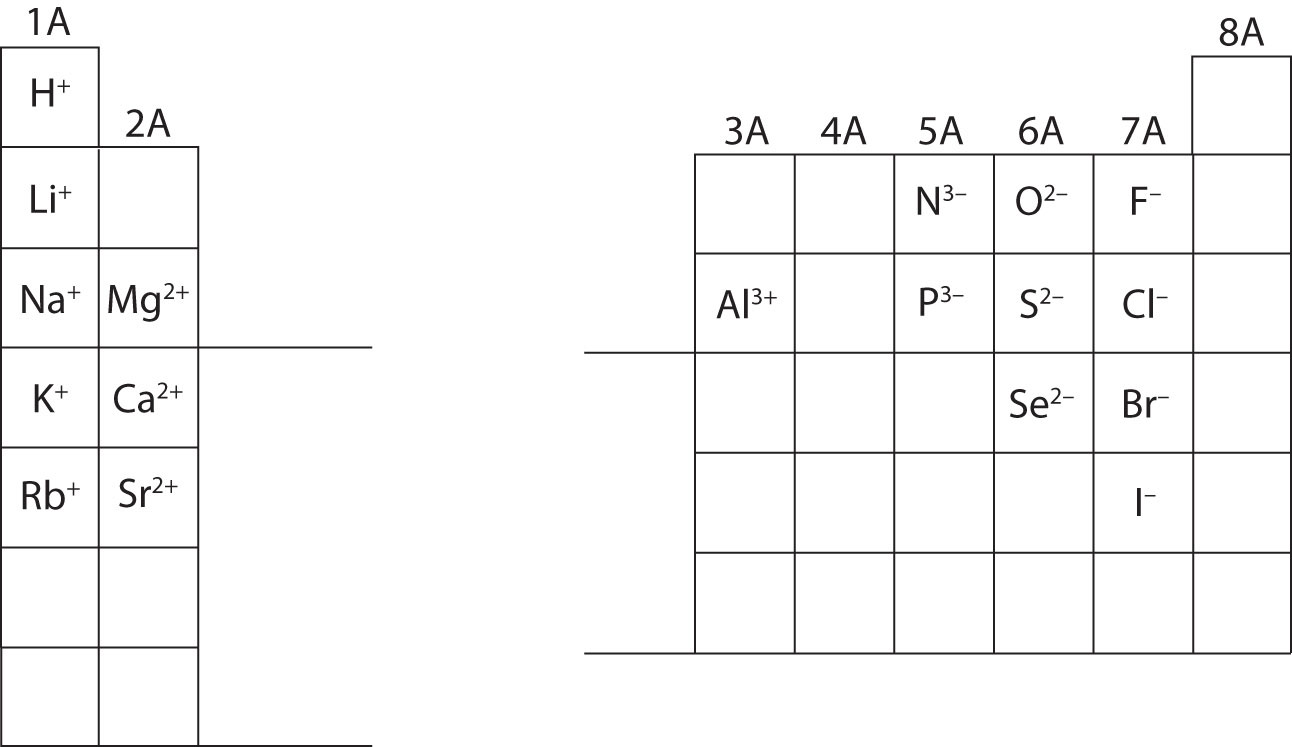
There are two lines of elements listed below the main table on the periodic chart the lanthanides and actinides. Full descriptions from write up sources. Valence electrons are the electrons present in the outermost shell of an atom.
Values in italics are predicted theoretical values. Actinides belong in period 7 group 3. The maximum number of valence electrons for an atom is eight.
Click on element atomic number element symbol element name and element valence electrons headers to sort. Determination of valence electrons. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search.
By looking at the group number that is given we can identify the. All lanthanides belong in period 6 group 3.

/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)



















/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)




0 Response to "Periodic Table Valence Electrons Chart"
Post a Comment