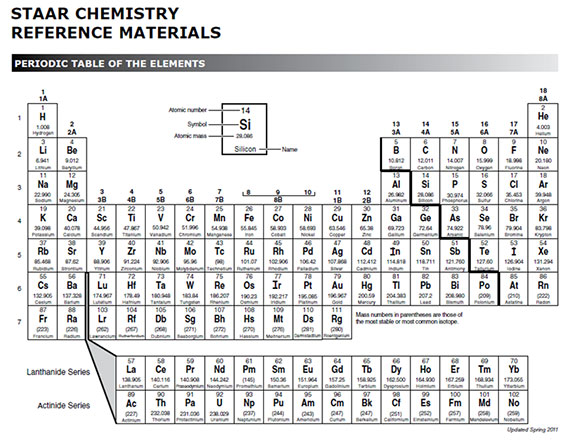
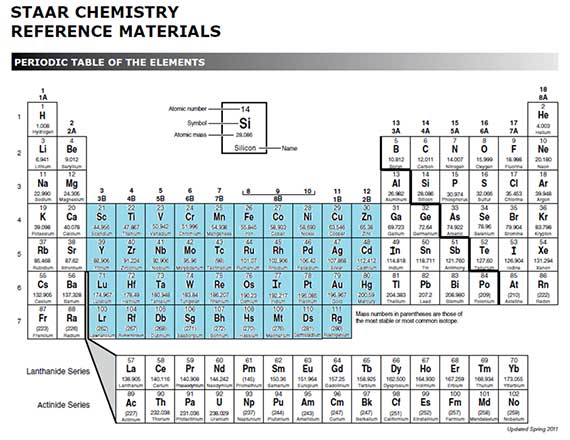
Periodic Table Valence Electrons
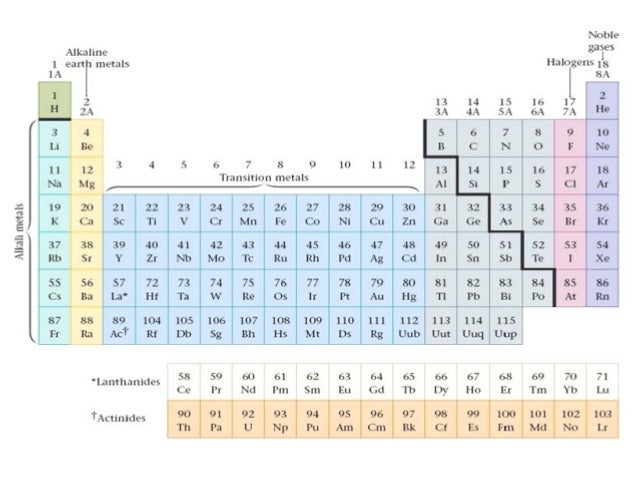
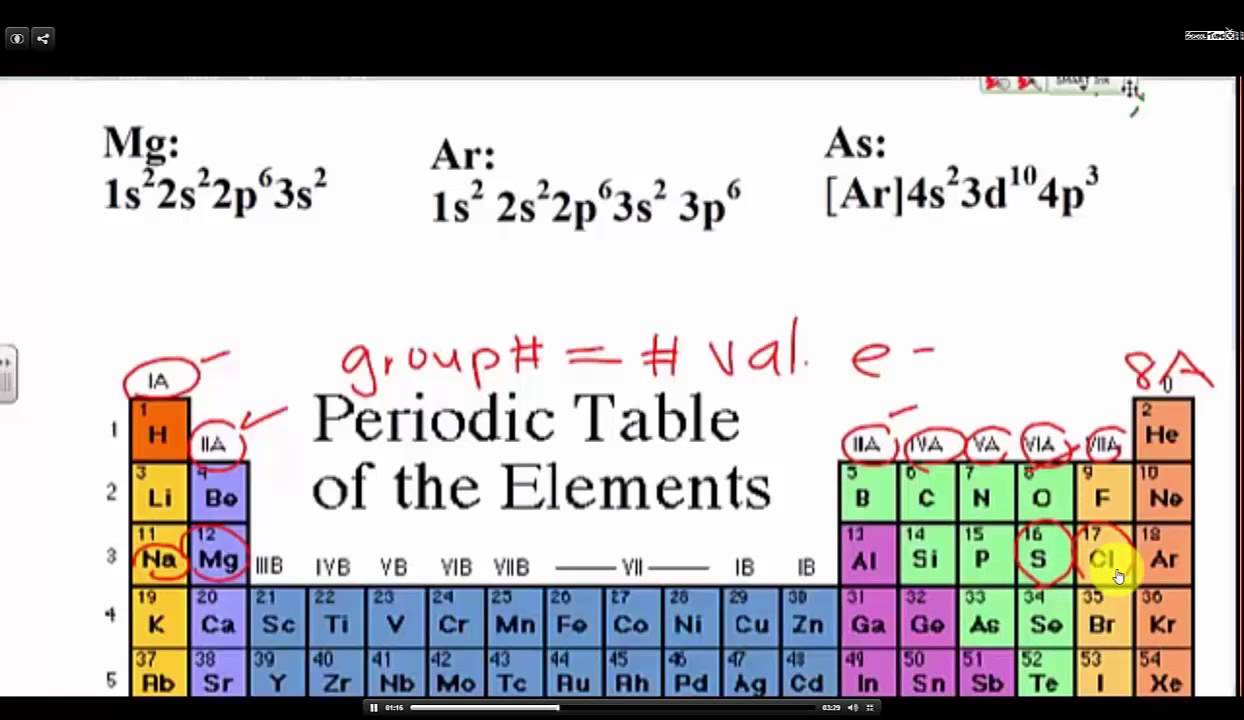
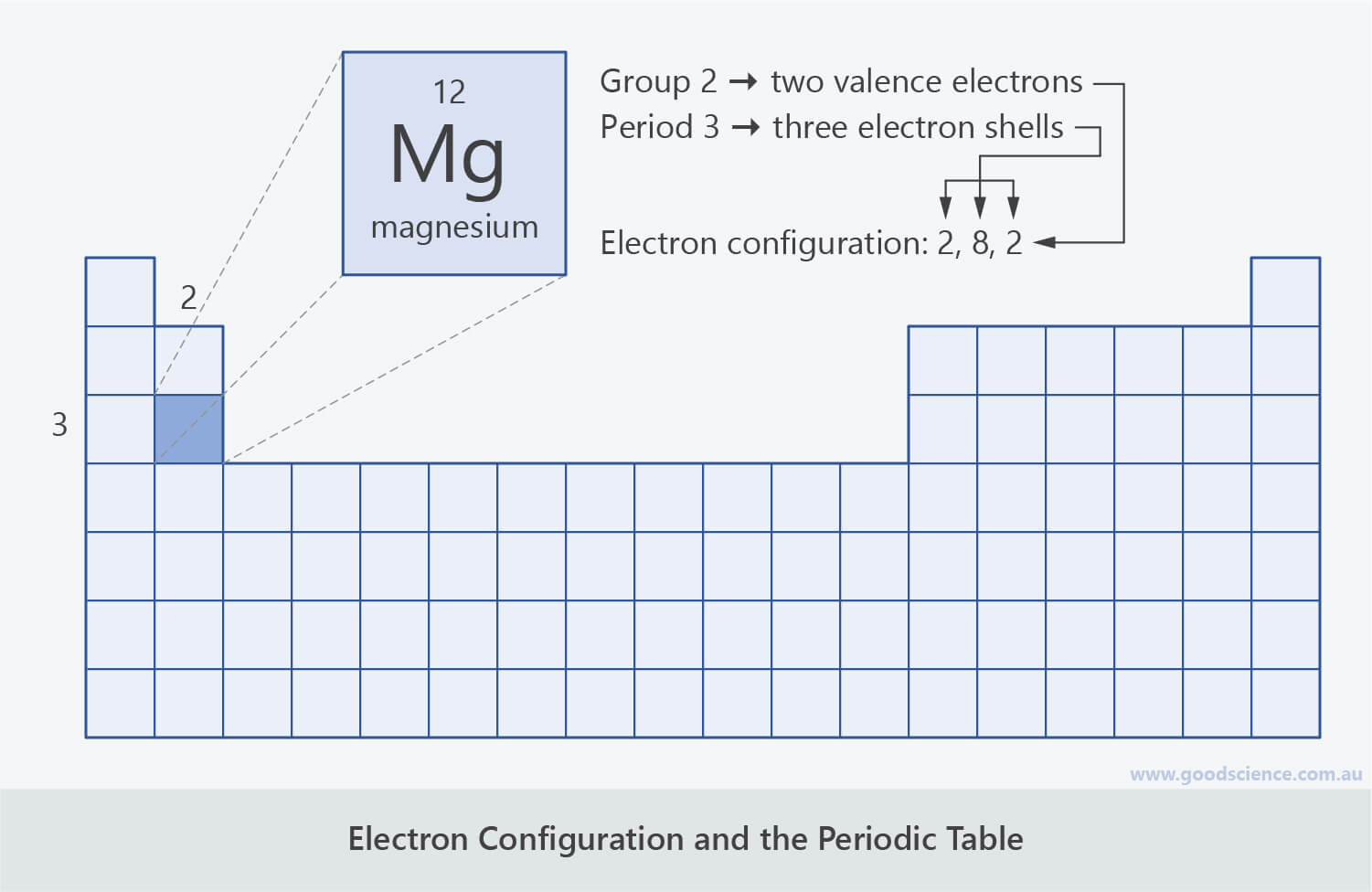
This information is available on a color periodic table of the elements or a black and white version. With the exception of groups 312 the transition metals the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column.
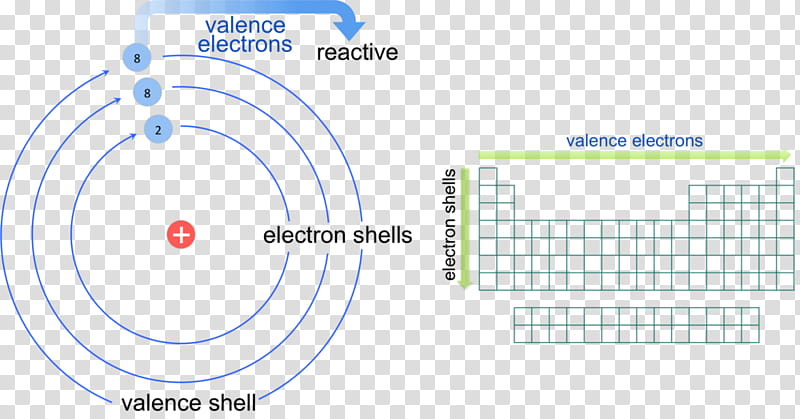
Valence electrons are the electrons present in the outermost shell of an atom.
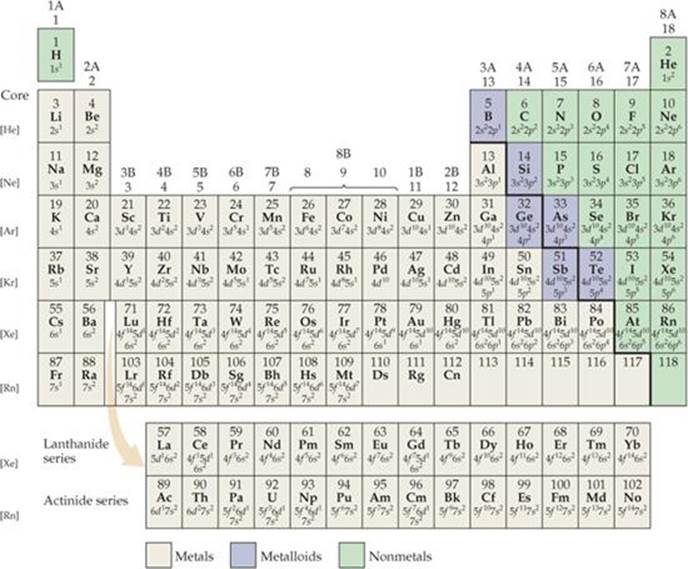
Periodic table valence electrons. The number of valence electrons. With the exception of groups 312 the transition metals the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under. Now locate the element that you want to find the valence electrons for on the table.
And so for this video were only talking about the valence electrons for elements in the main groups. Values in italics are predicted theoretical values. For example atoms in groups 1 and 2 have 1 and 2 valence electrons respectively.
The number of valence electrons of an element can be determined by the periodic table group vertical column in which the element is categorized. For facts physical properties chemical properties structure and atomic properties of the specific element click on the element symbol in the below periodic table. Remember that an elements electron cloud will become more stable by filling emptying or half filling the shell.
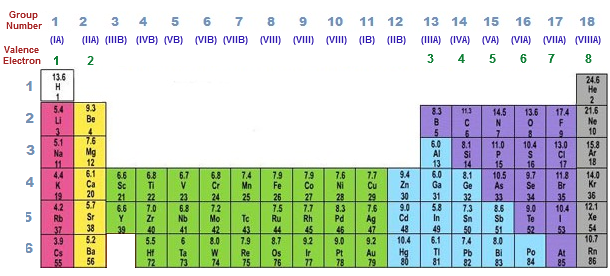
Periodic table of elements with valence electrons trends. Here is a table of element valences. Now that weve classified our elements into groups on the periodic table lets see how to determine the number of valence electrons.
You can easily determine the number of valence electrons an atom can have by looking at its group in the periodic table. The most common valences are in bold. A simpler version listing only the most common valence charges is also available.
This is a table of the valences or oxidation states of the elements. You can do this with its chemical symbol the letters in each box its atomic number the number in the top left of each box or any of the other pieces of information available to you on the table. In the below periodic table you can see the trend of valence electrons.
The number of valence electrons of an element can be determined by the periodic table group vertical column in which the element is categorized. You can use information from the periodic table to find the number of valence electrons. Click here to buy a book photographic periodic table poster card deck or 3d print based on the images you see here.
By definition valence electrons travel in the subshell farthest away from the nucleus of the atom. Also shells dont stack neatly one on top of another so dont always assume an elements valence is determined by the number of electrons in its outer shell.



























0 Response to "Periodic Table Valence Electrons"
Post a Comment