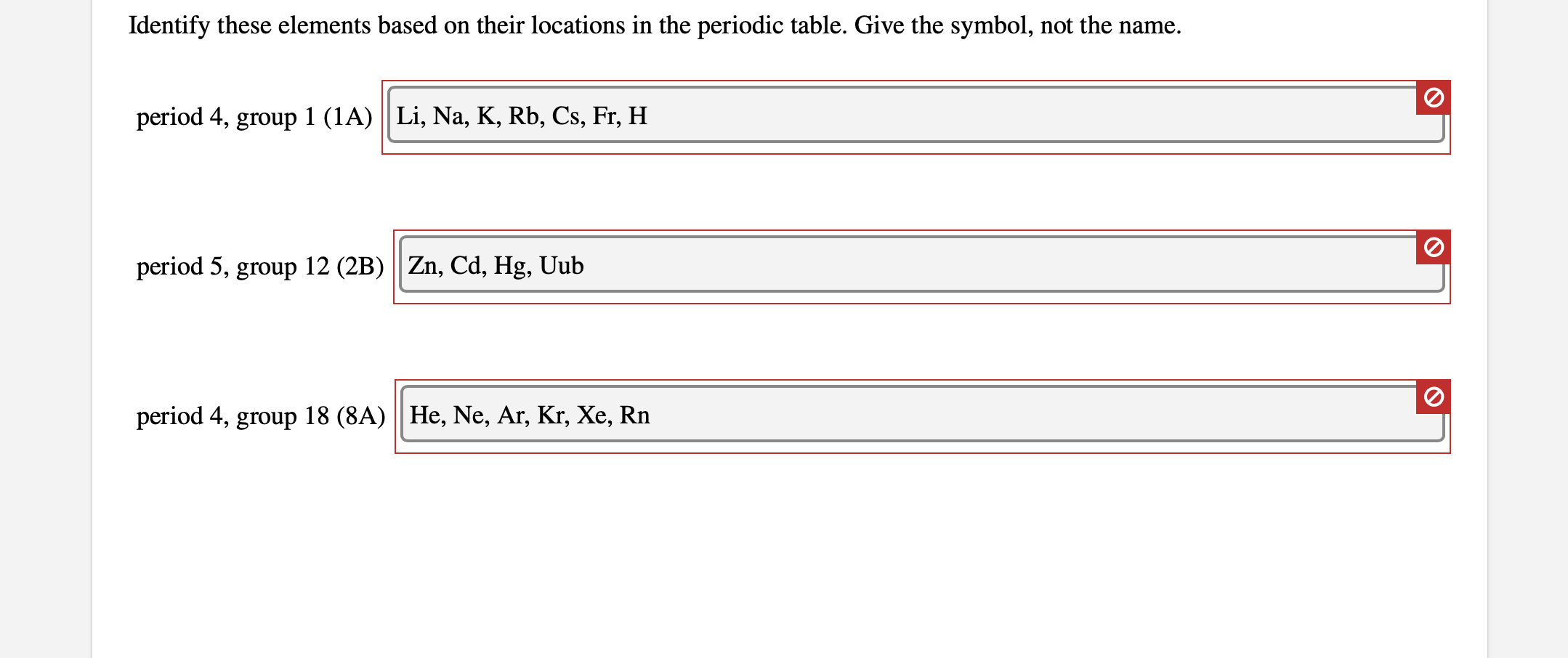
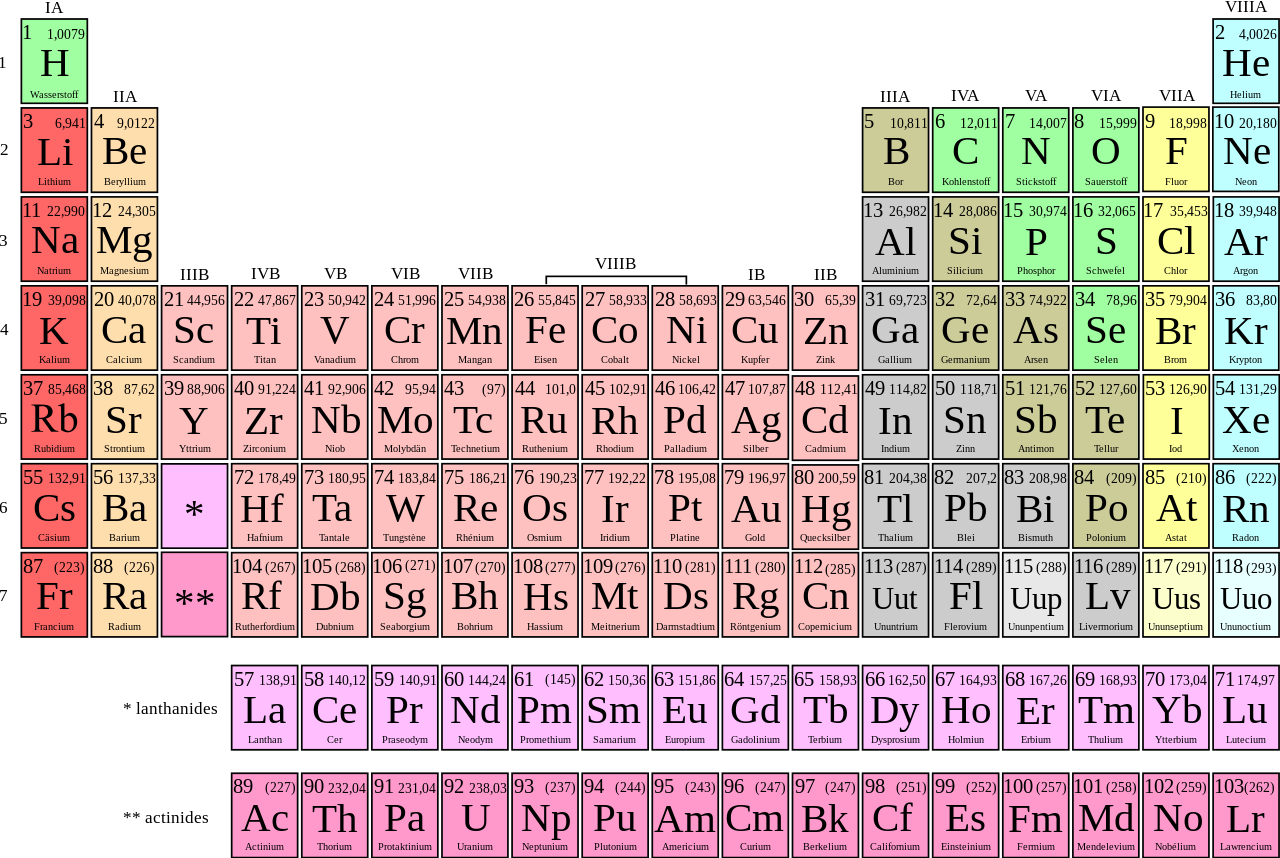
Periodic Table Groups And Periods Names
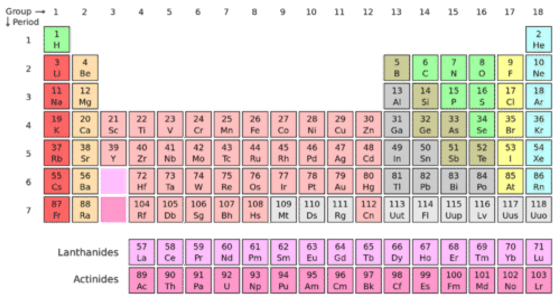
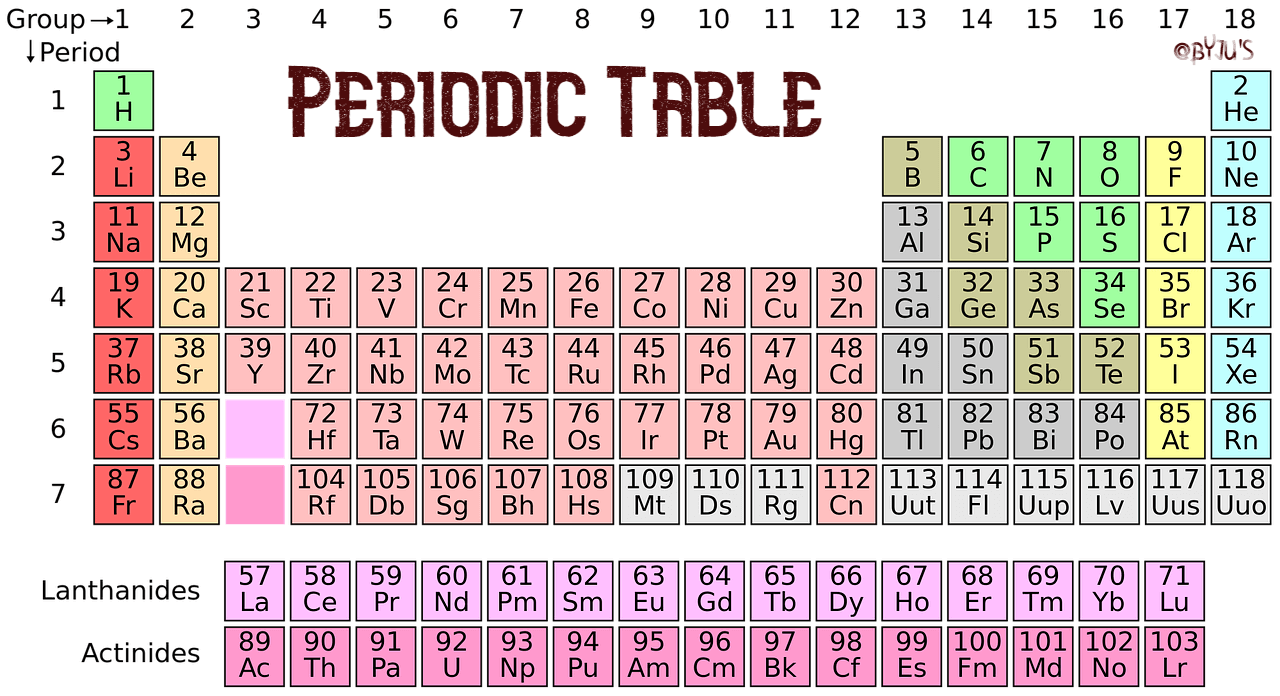
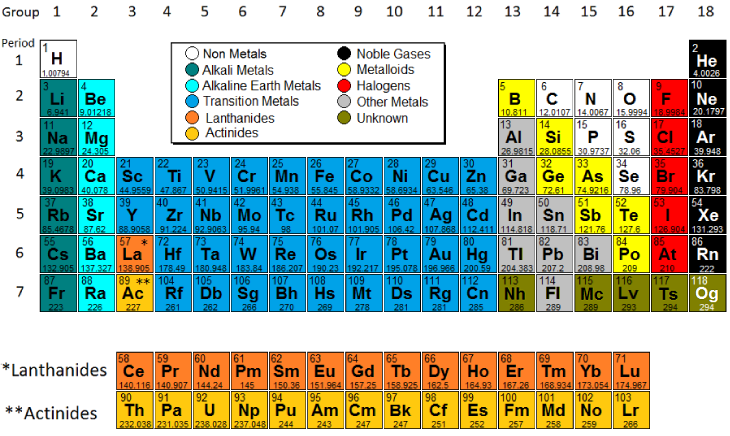
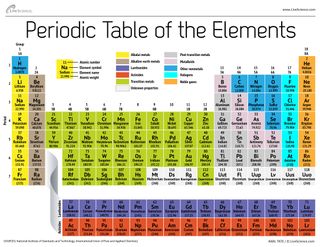
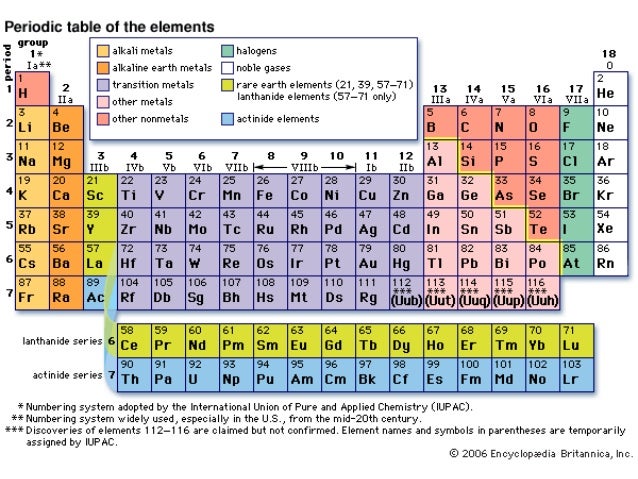
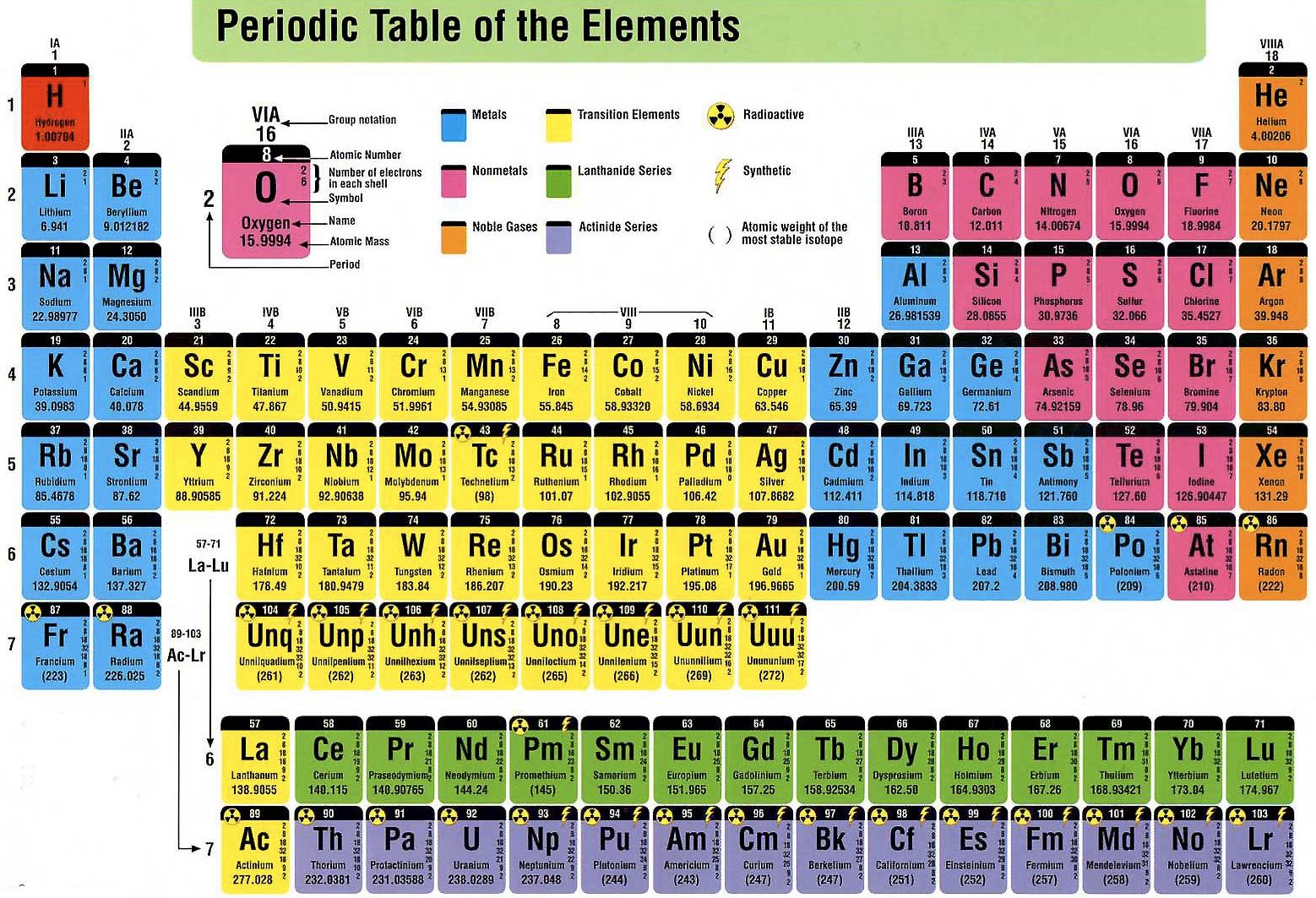
A group is a vertical column of the periodic table based on the organization of the outer shell electrons. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesthe structure of the table shows periodic trendsthe seven rows of the table called periods generally have metals on the left and nonmetals on the right.
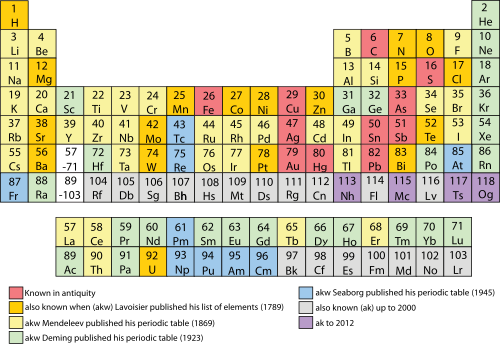
The names on the periodic table of mendeleev are the names of chemical elements.

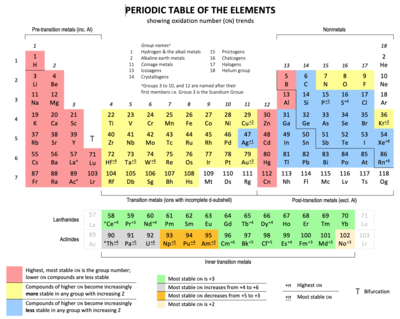
Periodic table groups and periods names. Groups 1 2 except hydrogen and 13 18 are termed main group elements. Groups 3 11 are termed transition elements. Later 1902 mendeleev accepted the evidence for their existence and they could be placed in a new group 0 consistently and without breaking the periodic table principle.
Most elements are metals. R group name as recommended by iupac. Periods in the periodic table in each period horizontal row the atomic numbers increase from left.
The vertical columns of elements are called groups or families. Groups 1 2 termed s block elements. C group 18 the noble gases were not discovered at the time of mendeleevs original table.
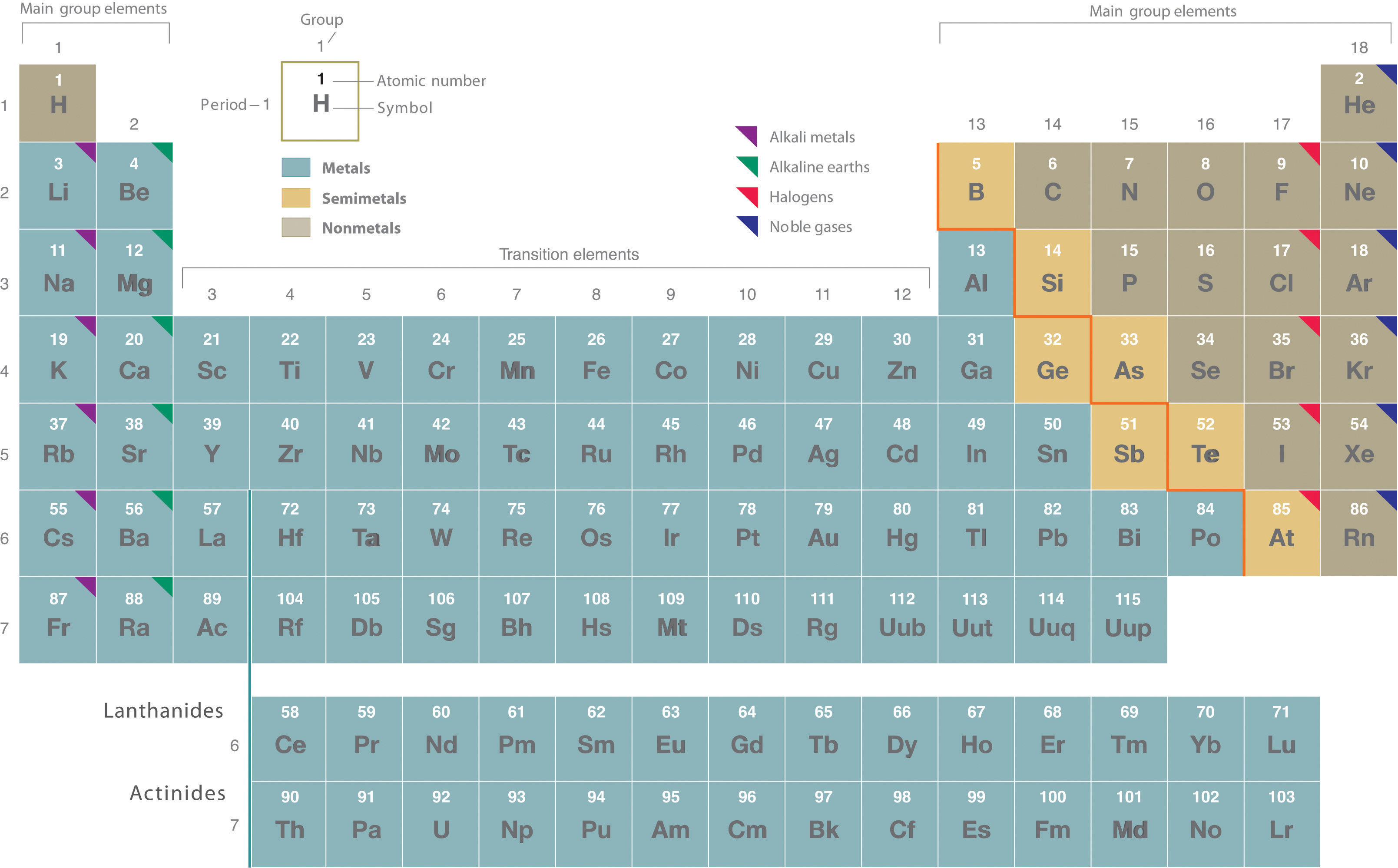
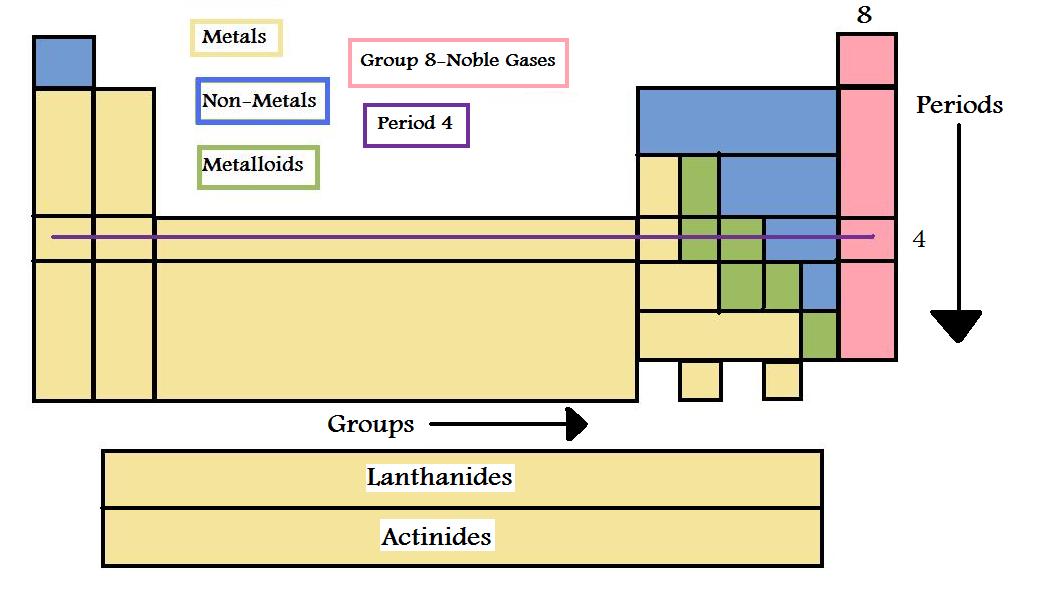
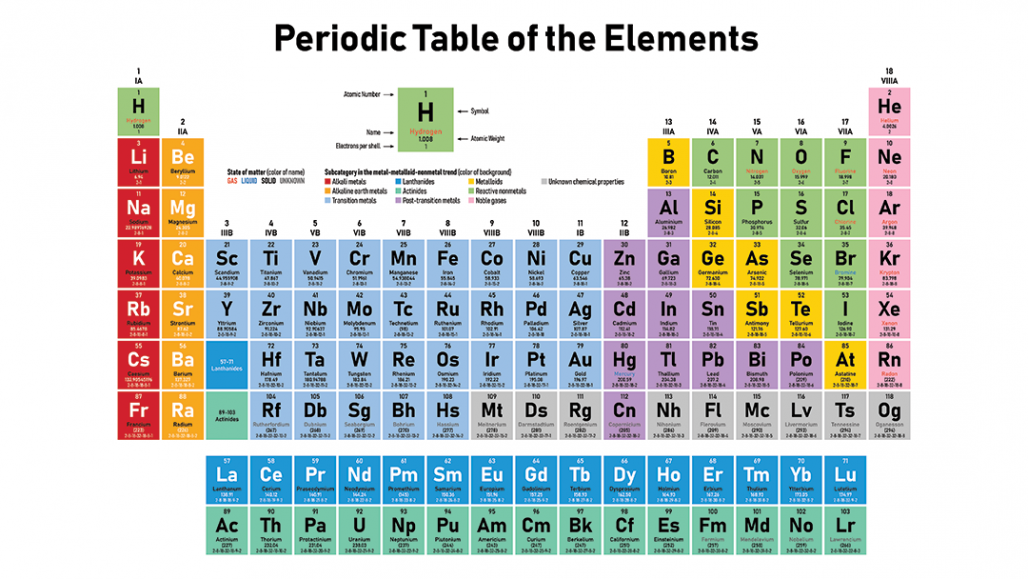
They are pulled out in order to make the table itself fit more easily onto a single page. Asked in physics chemistry history of science periodic table how many groups does the periodic table contain. The most common way the periodic table is classified by metals nonmetals and metalloids.
There are a. Periods 6 and 7 have 32 elements because the two bottom rows that are separated from the rest of the table belong to those periods. Atomic number increases as you move down a group or across a period.
Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows across the periodic table while groups are vertical columns down the table. Groups 3 12 are termed d block elements.
One reason the periodic table of the elements is so useful is that it is a means of arranging elements according to their similar properties. There are multiple ways of grouping the elements but they are commonly divided into metals semimetals metalloids and nonmetals. In the periodic table of elements there are seven horizontal rows of elements called periods.
The group number is an identifier used to describe the column of the standard periodic table in which the element appears. Groups and periods the periodic table is a way of arranging the elements so patterns in their properties and reactions can be identified and explained. This is what is meant by periodicity or periodic table trends.

:max_bytes(150000):strip_icc()/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)






:max_bytes(150000):strip_icc()/PeriodicTableallcolor-58b5d9293df78cdcd8d043b6.jpg)

:max_bytes(150000):strip_icc()/the-periodic-table--digital-illustration--73016803-598b218ec41244001024af78.jpg)

/Periodic-Table-Metals-56a12db33df78cf772682c44.png)









0 Response to "Periodic Table Groups And Periods Names"
Post a Comment