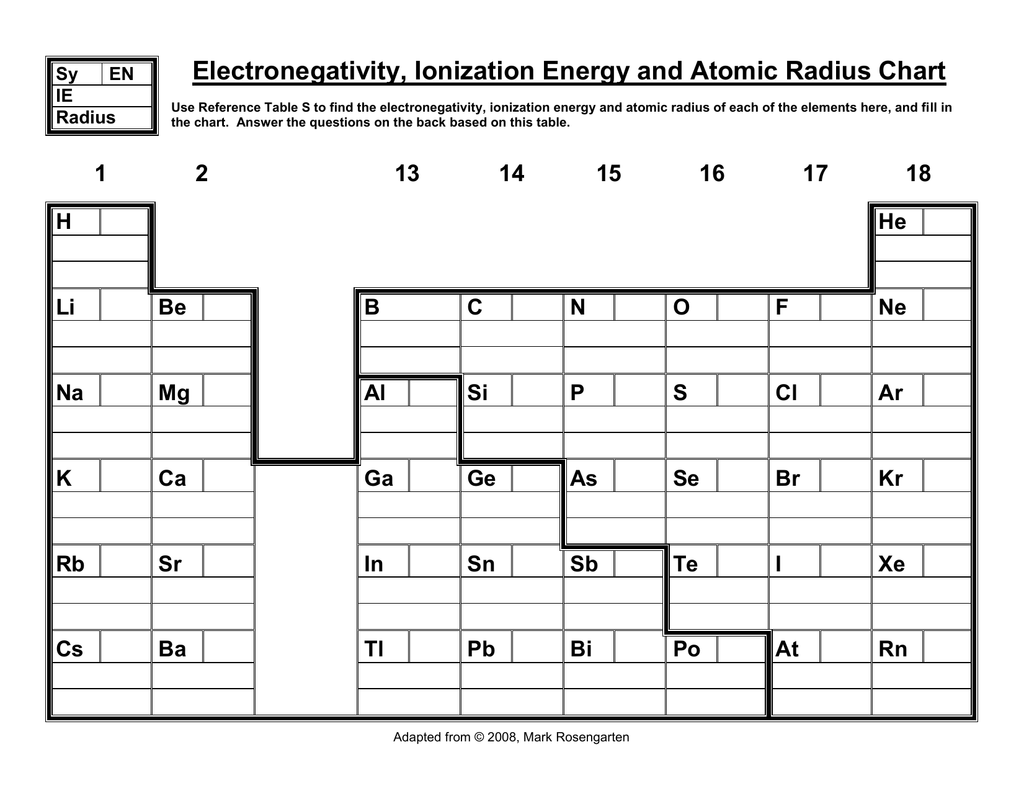
Periodic Table Electronegativity Diagram
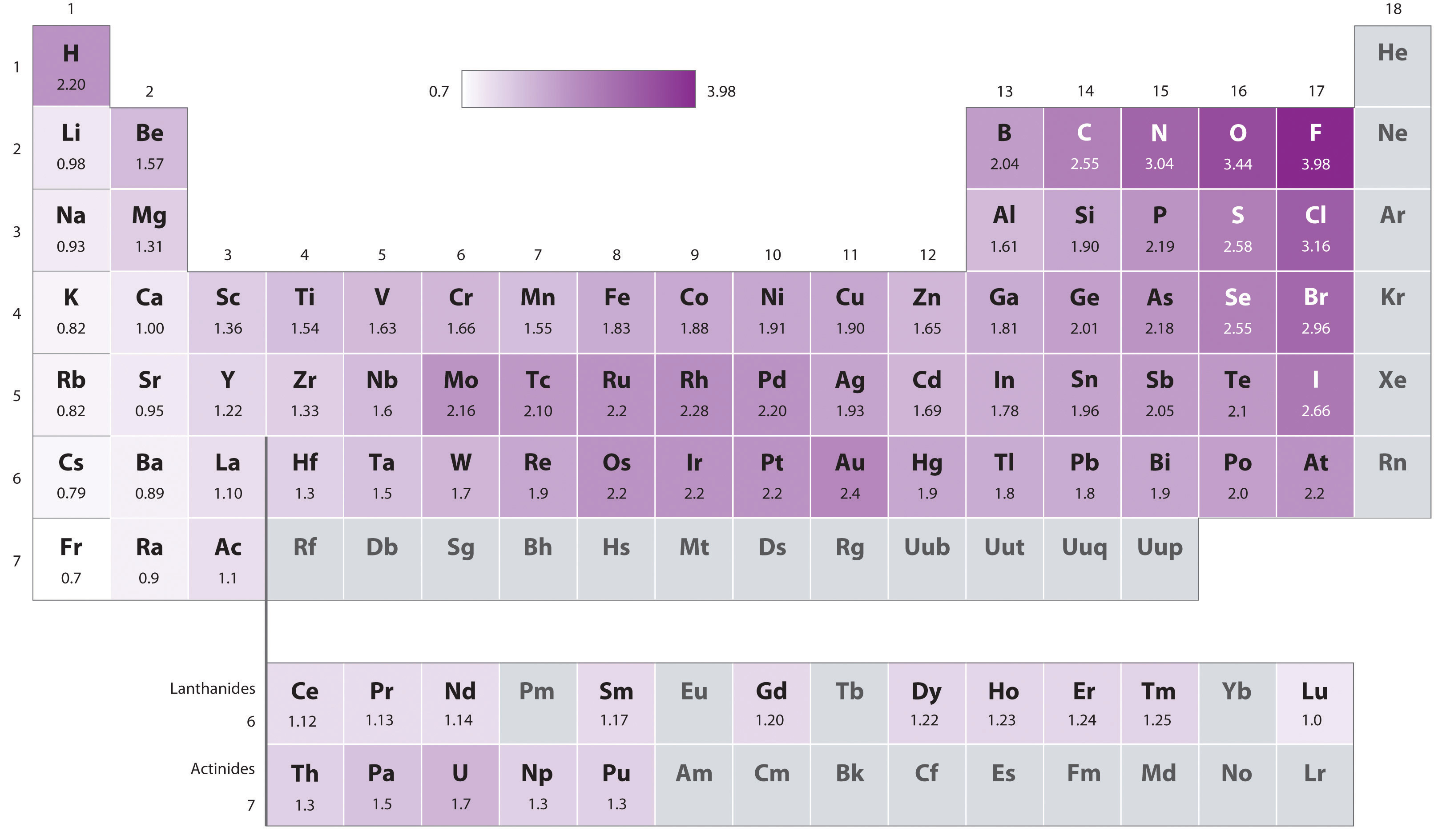
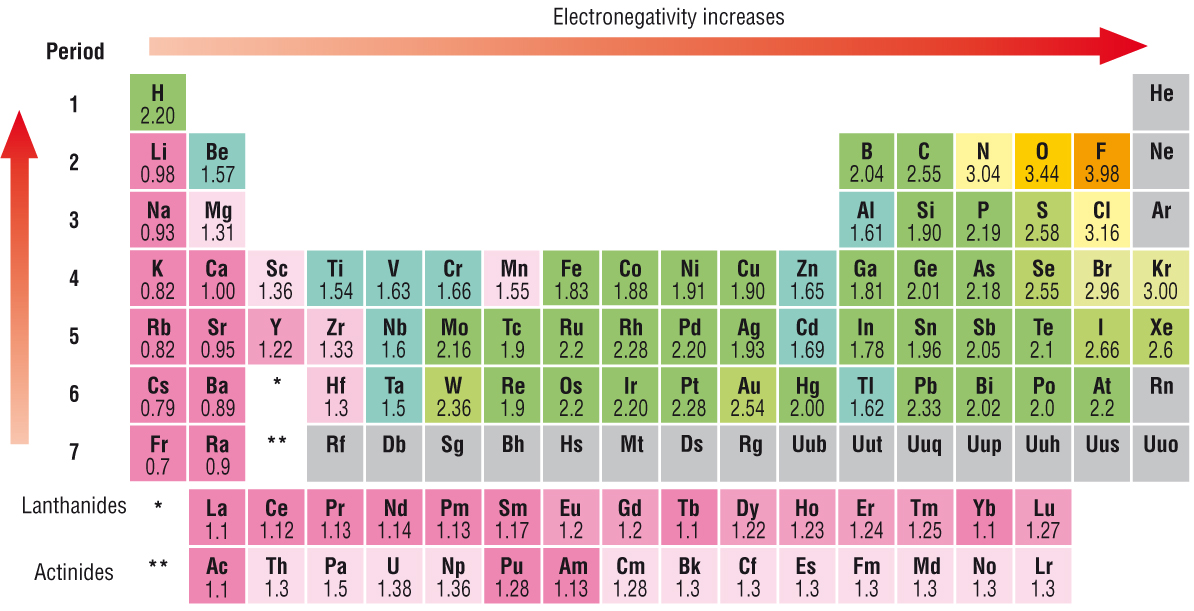
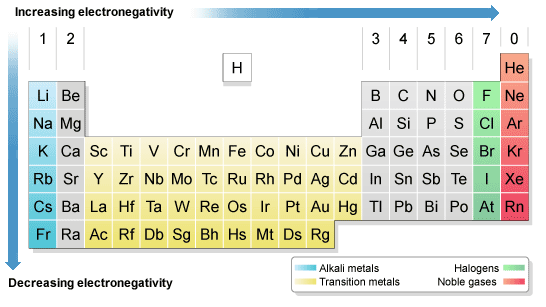
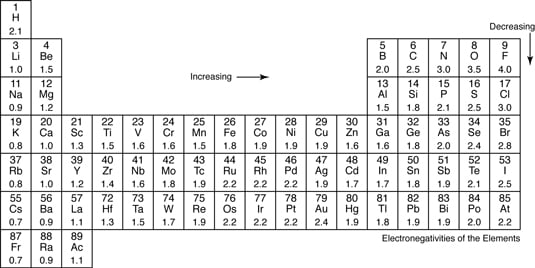
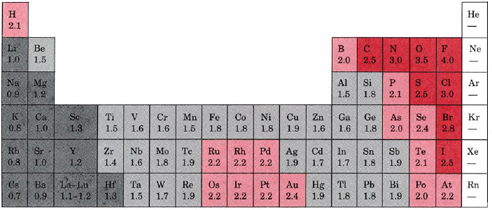
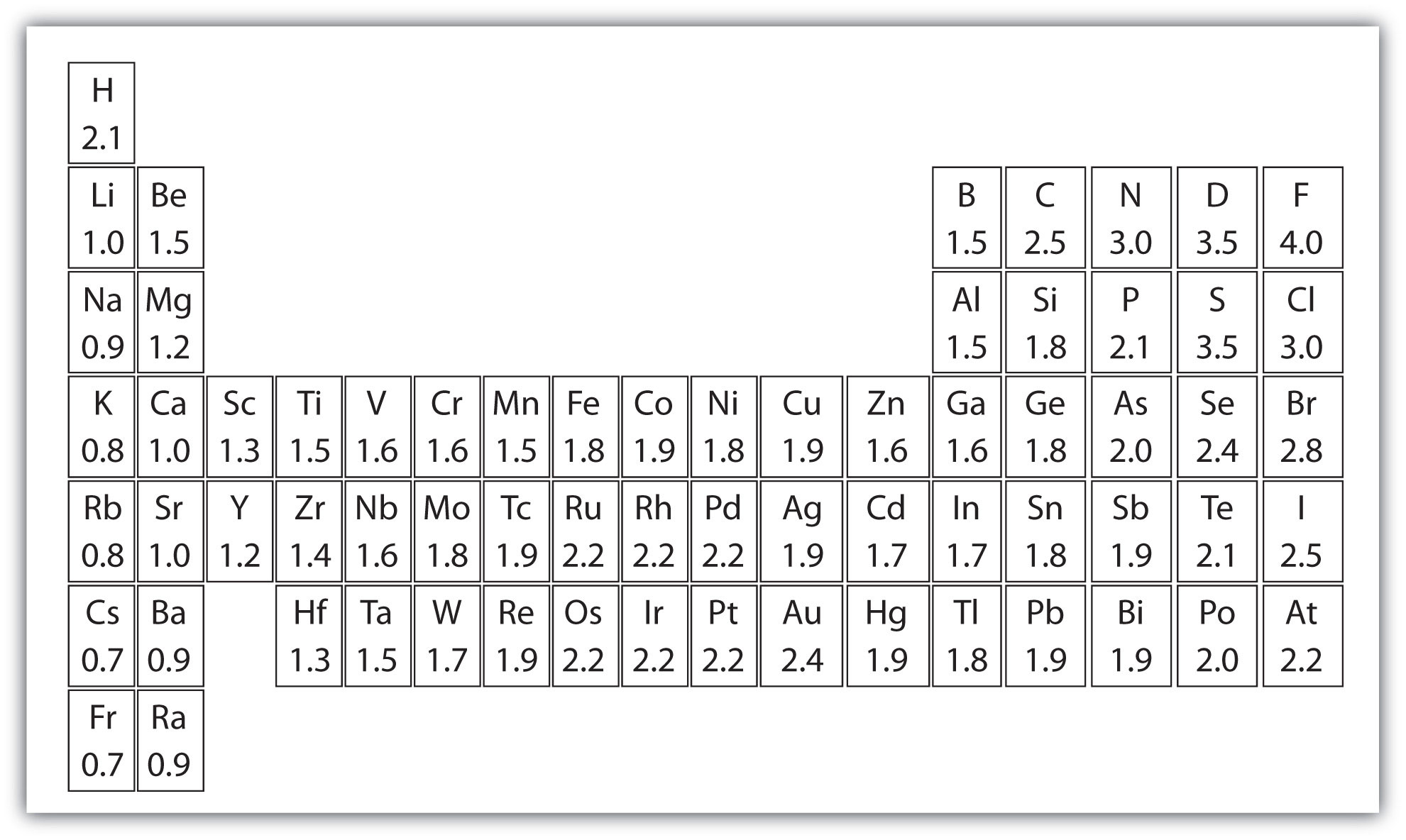
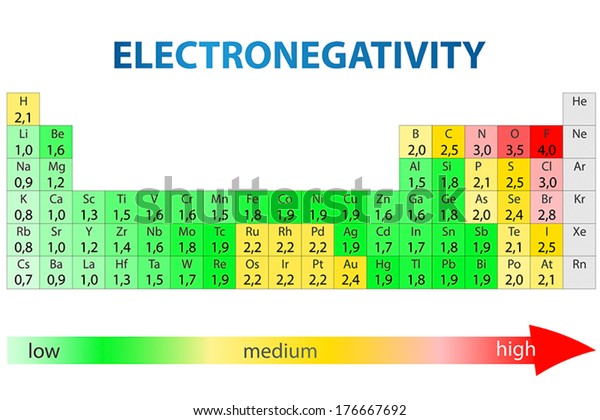
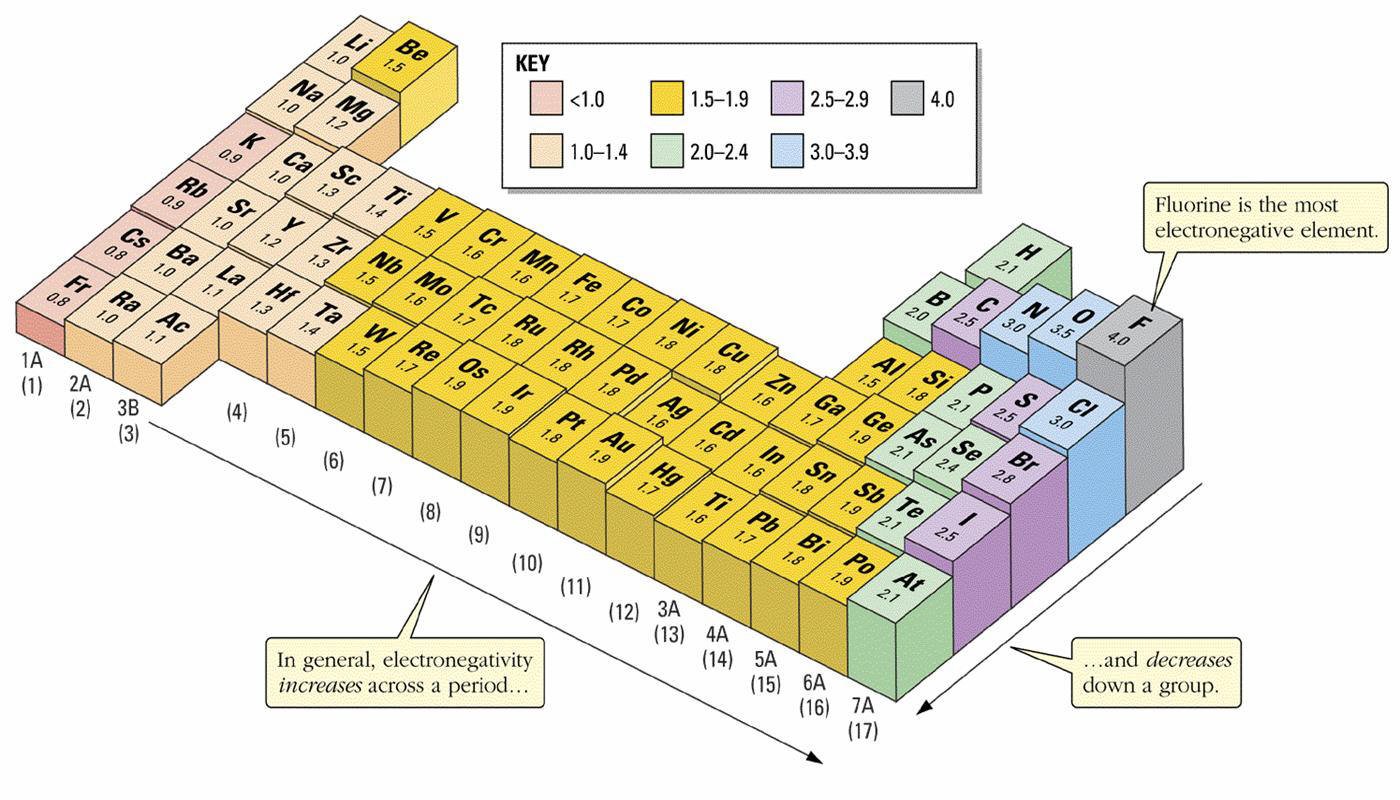
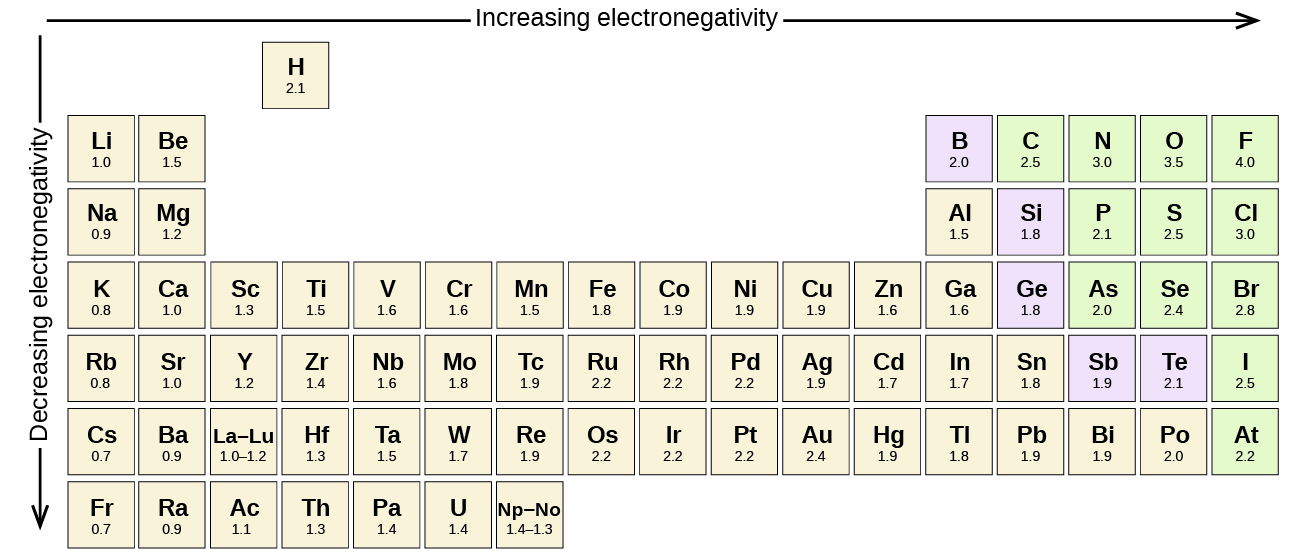
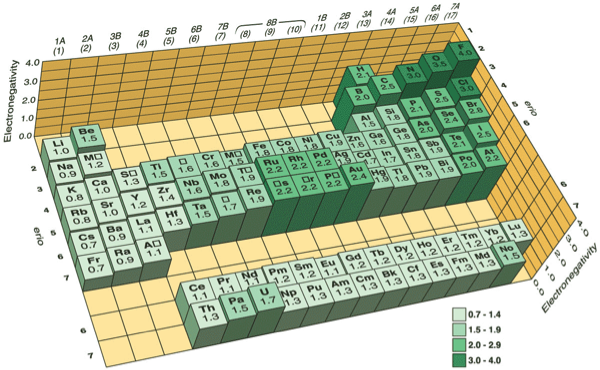
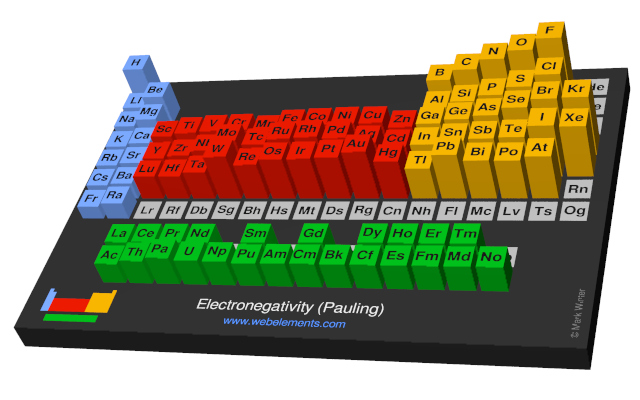
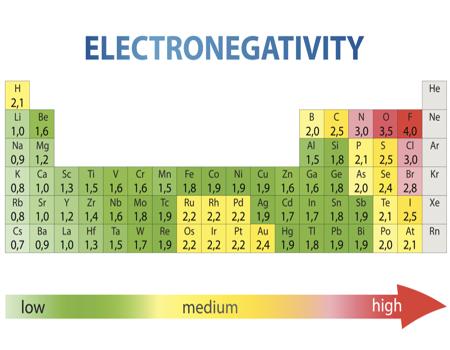
Keep in mind that electronegativities are approximate measures of the relative tendencies of these elements to attract electrons to themselves in a chemical bond. If you remember that fact everything becomes easy because electronegativity must always increase towards fluorine in the periodic table.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structurethe chemical symbol for hydrogen is h.
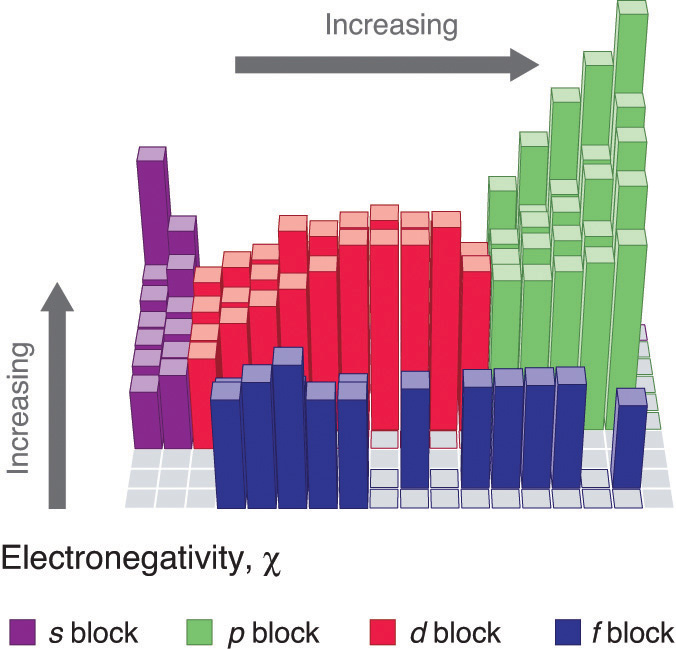
Periodic table electronegativity diagram. Full descriptions from write up sources. This simplification ignores the noble gases. Electronegativity reflects how easily an atom can form a chemical bond.
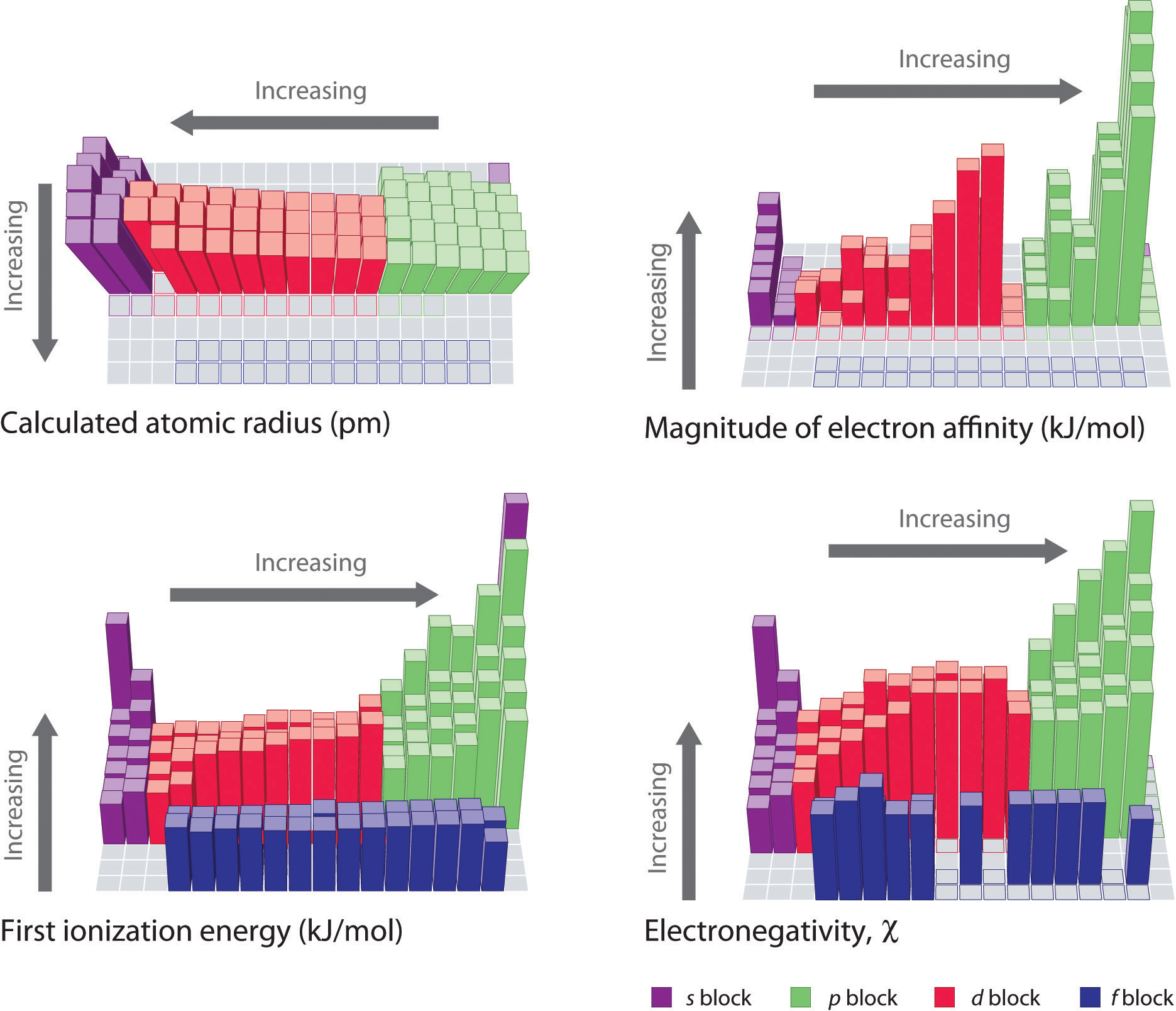
When one moves from top to bottom the atomic radius increases the ionization energy decreases and the electronegativity too decreases. The electronegativity chart describes how atoms can attract a pair of electrons to itself by looking at the periodic table you can identify and determine electronegativity values of elements from 0 to 4. Historically this is because they were believed not to form bonds and if they dont form bonds they cant have an electronegativity value.
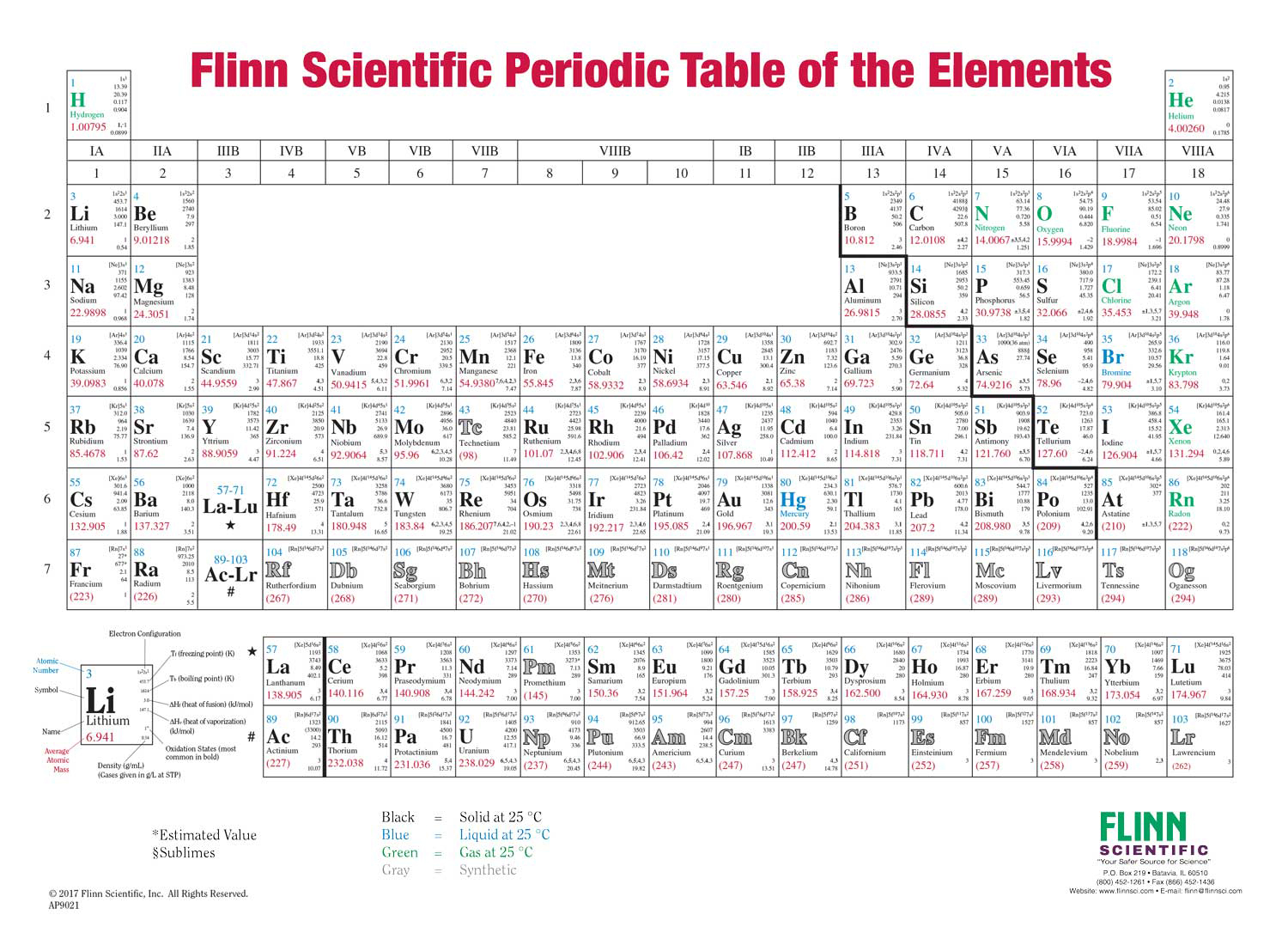
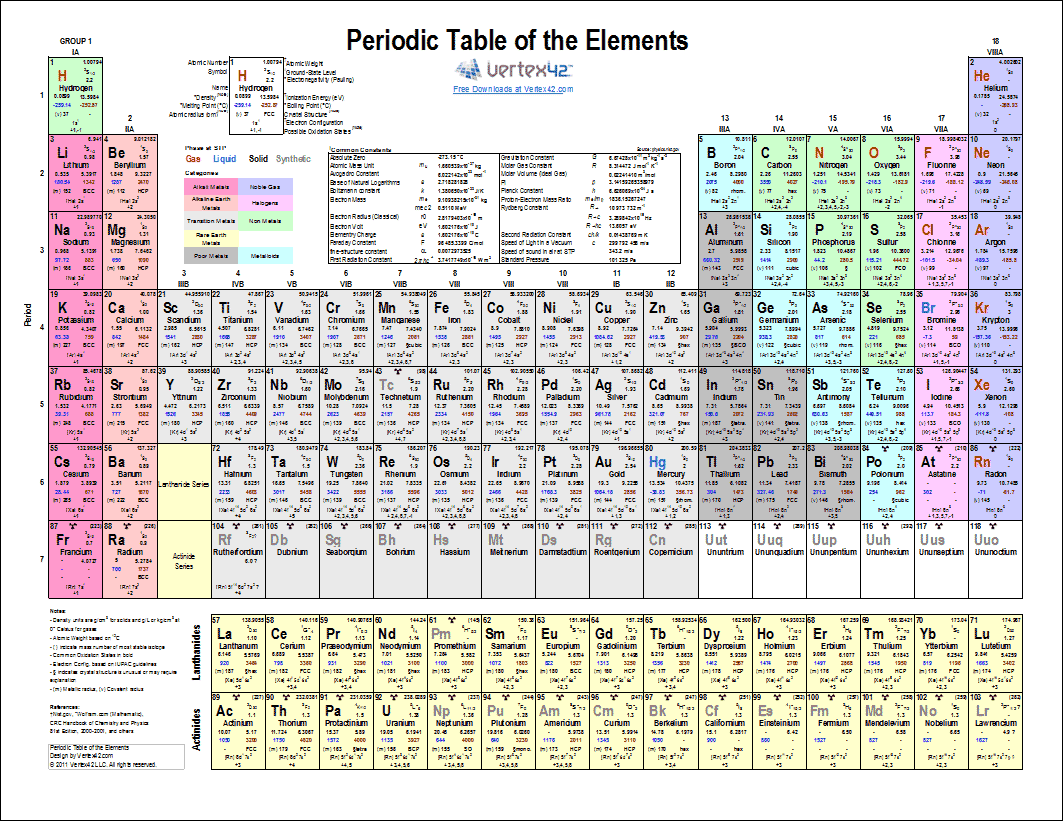
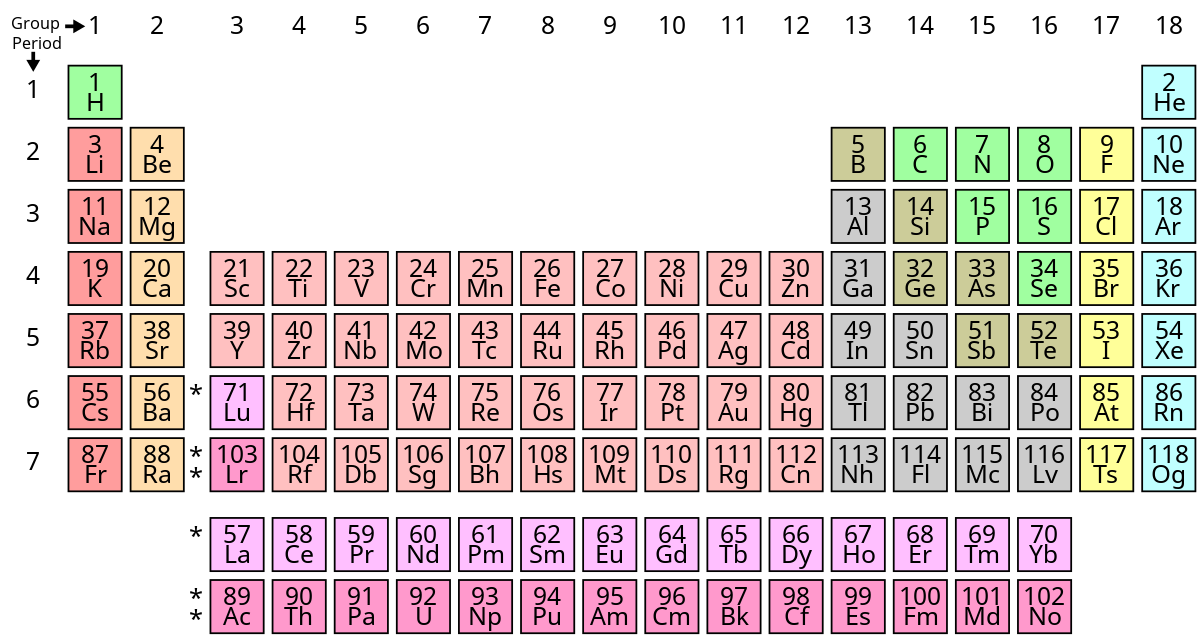
The structure of the table shows periodic trends. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search. The periodic table contains a lot more information than merely the names of each of the chemical elements.
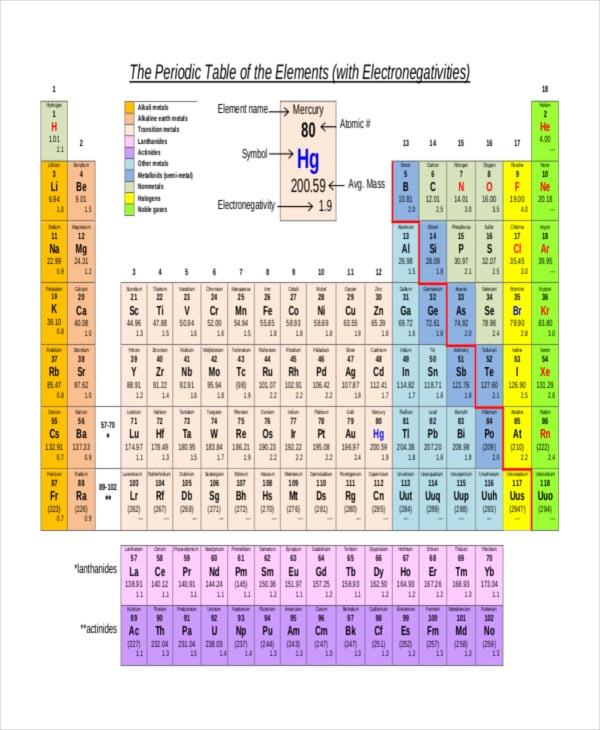
The periodic table of the elements with electronegativities 1 18 hydrogen 1 h 101 21 2 alkali metals alkaline earth metals transition metals lanthanides actinides other metals metalloids semi metal nonmetals 694 halogens noble gases element name 80 symbol beryllium electronegativity mercury hg 20059 19 atomic lithium avg. When one moves left to right in the periodic table the atomic radius of the elements decreases ionization energy increases and electronegativity increases. A key piece of information they contain is the electronegativity value of each of.
Keep in mind the noble gasses column at the right hand side of the periodic table are relatively inert so their electronegativity approaches zero exception to the overall trend. The larger the difference between electronegativity values the more likely two atoms are to form a chemical bond. Its monatomic form h is the most abundant chemical substance in the universe constituting roughly 75 of all baryonic mass.
Mass 13 14. Generally electronegativity increases from left to right and decreases as you move down a group. When you take a closer look at a periodic table that includes the different electronegativity numbers for each specific element of the periodic table youll notice that when you are moving from the left to the right the atomic radius of the elements decreases.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. The greater an atoms electronegativity the greater its ability to attract electrons to itself. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical properties.
There are particular electronegativity trends that are seen in the periodic table. Periodic table with electronegativity. Nevertheless the ionization increases.







:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)

/PeriodicTableElectronegativity-56a12a045f9b58b7d0bca77c.jpg)




:max_bytes(150000):strip_icc()/PeriodicTableElectronegativity-56a12a045f9b58b7d0bca77c.jpg)


0 Response to "Periodic Table Electronegativity Diagram"
Post a Comment