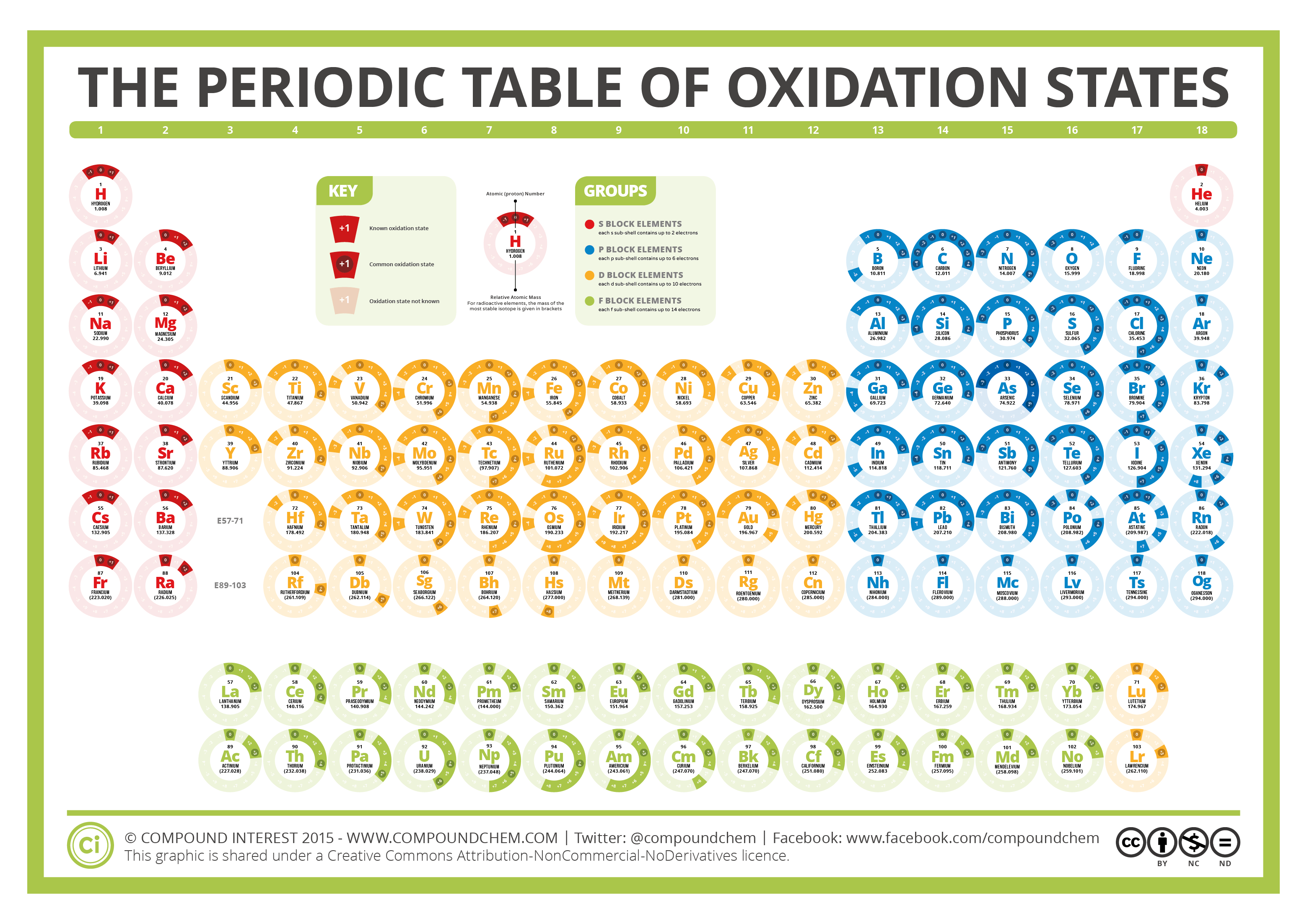
Periodic Table Charges Ions
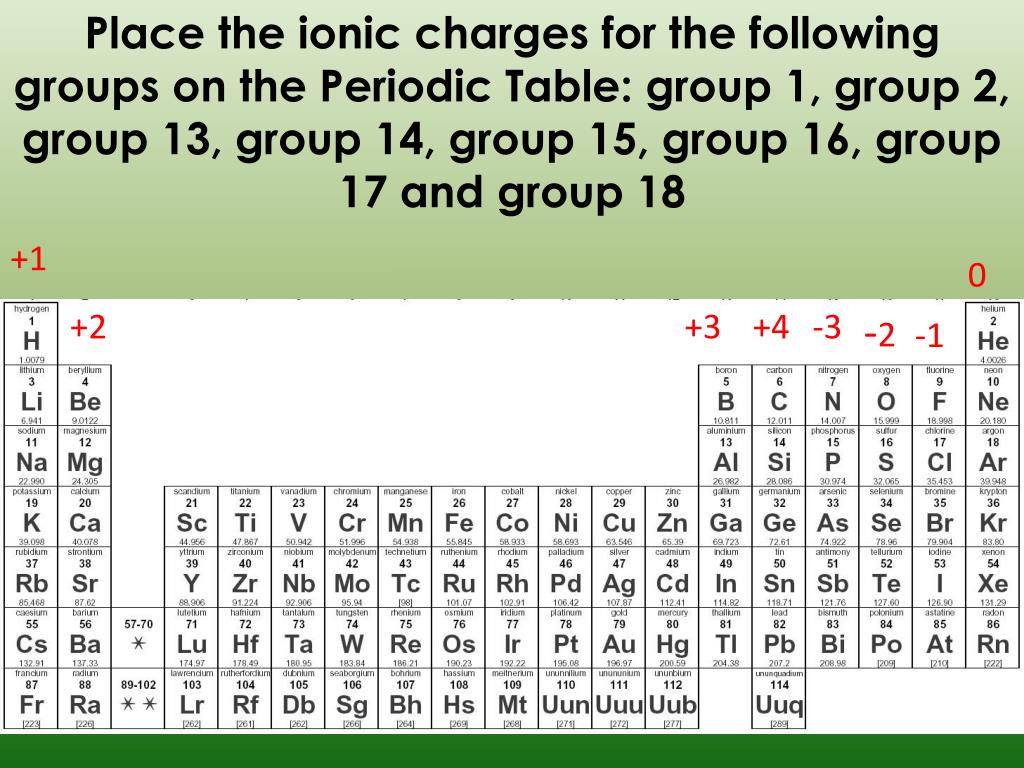
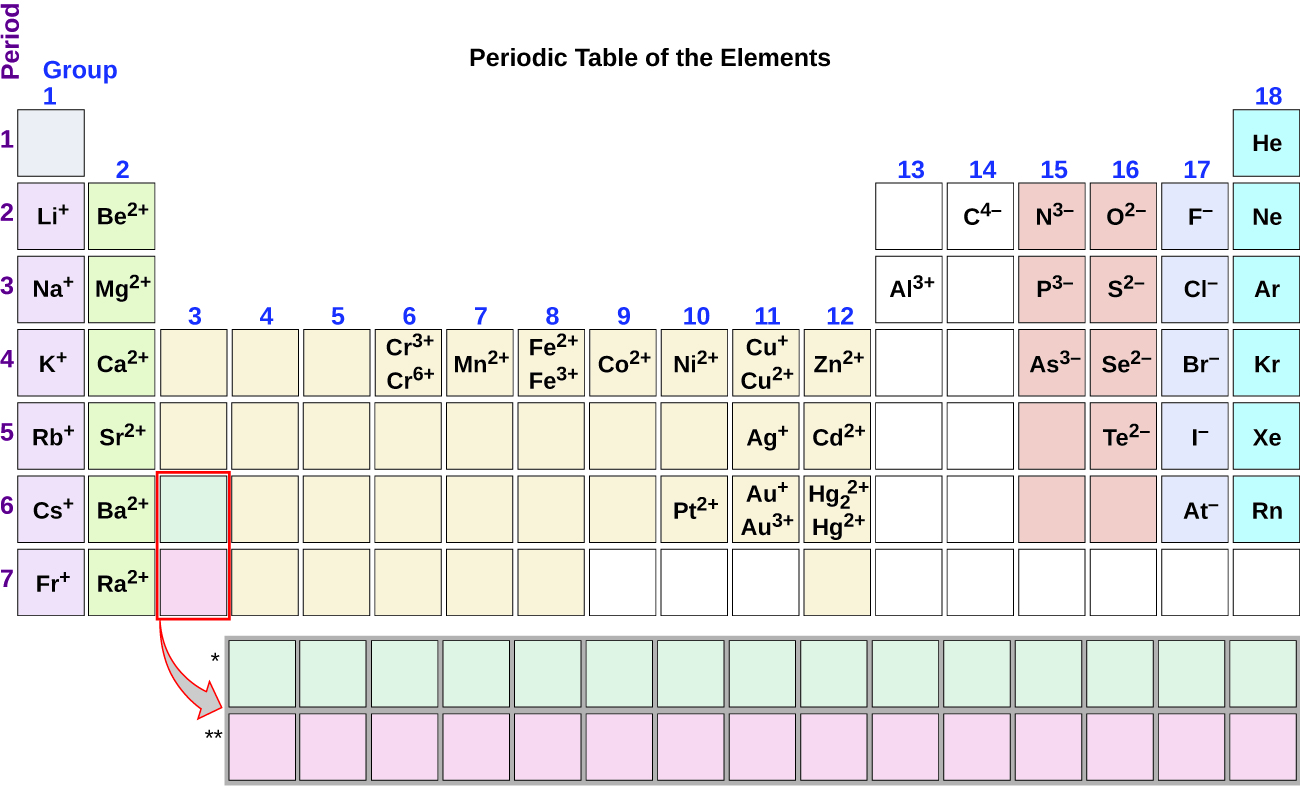
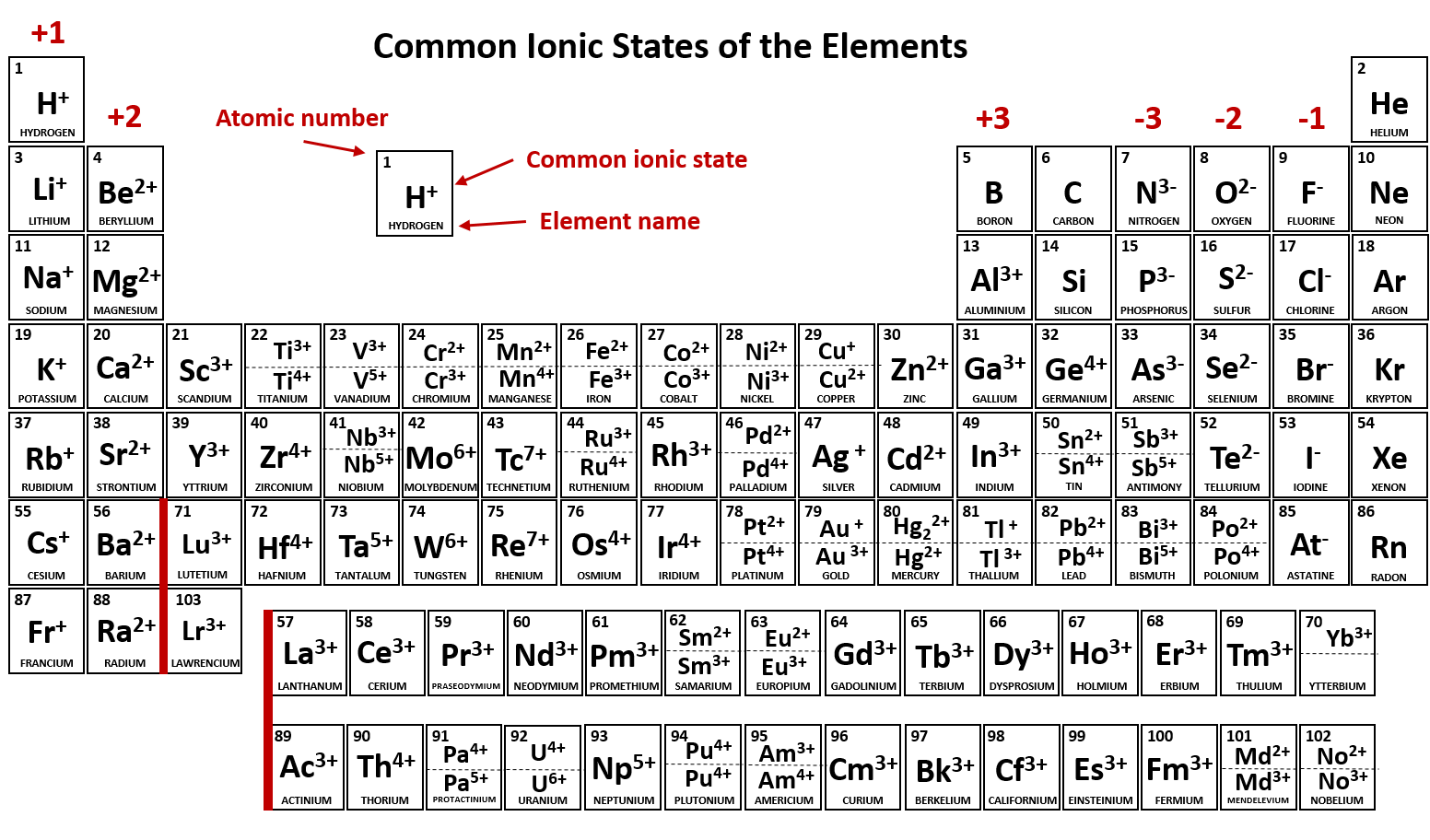
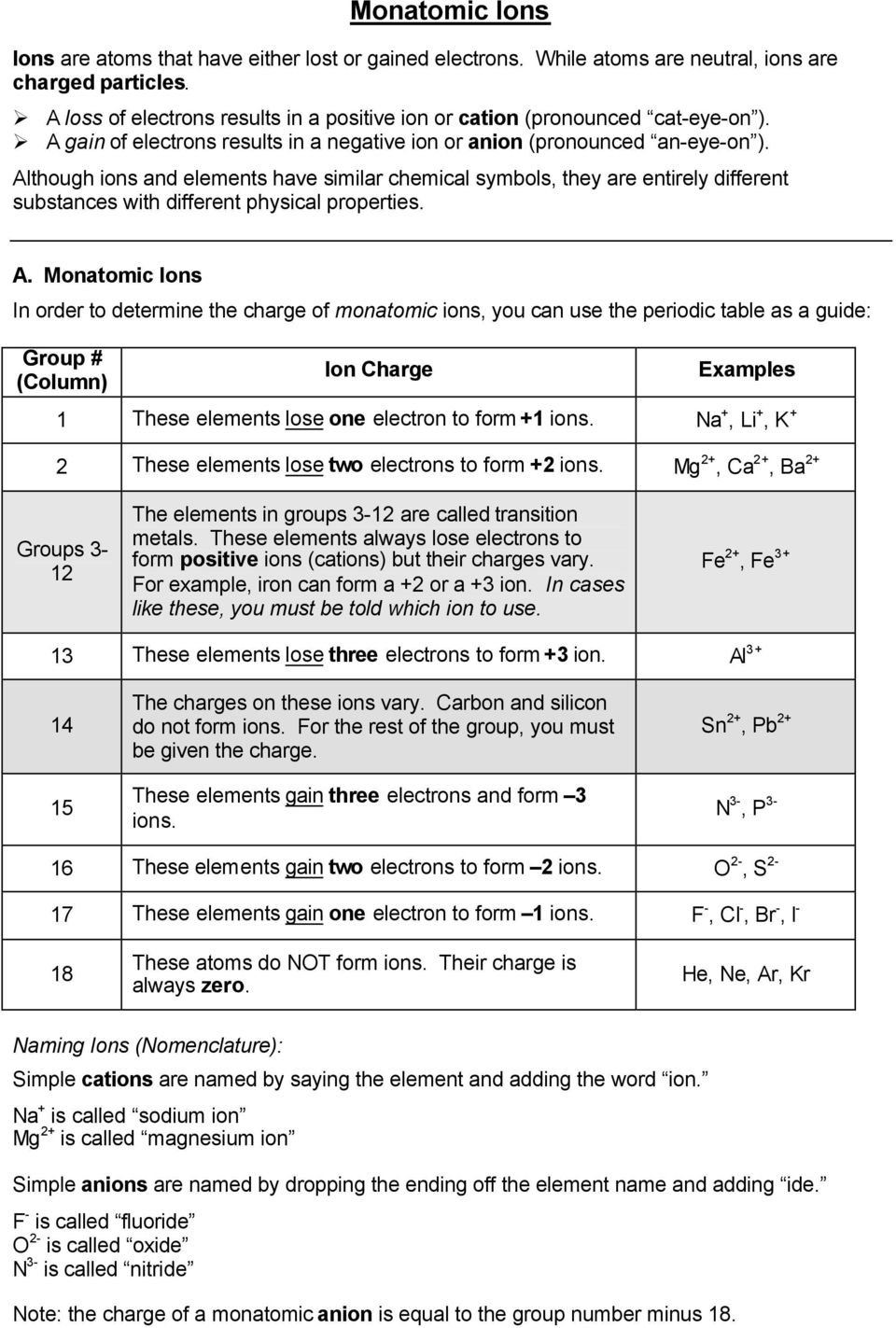
Briefly speaking the charge of an element in its ionic form refers to the actual number of electrons that it loses or gains to achieve the nearest noble gas configuration. Metal ions may have other charges or oxidation states.
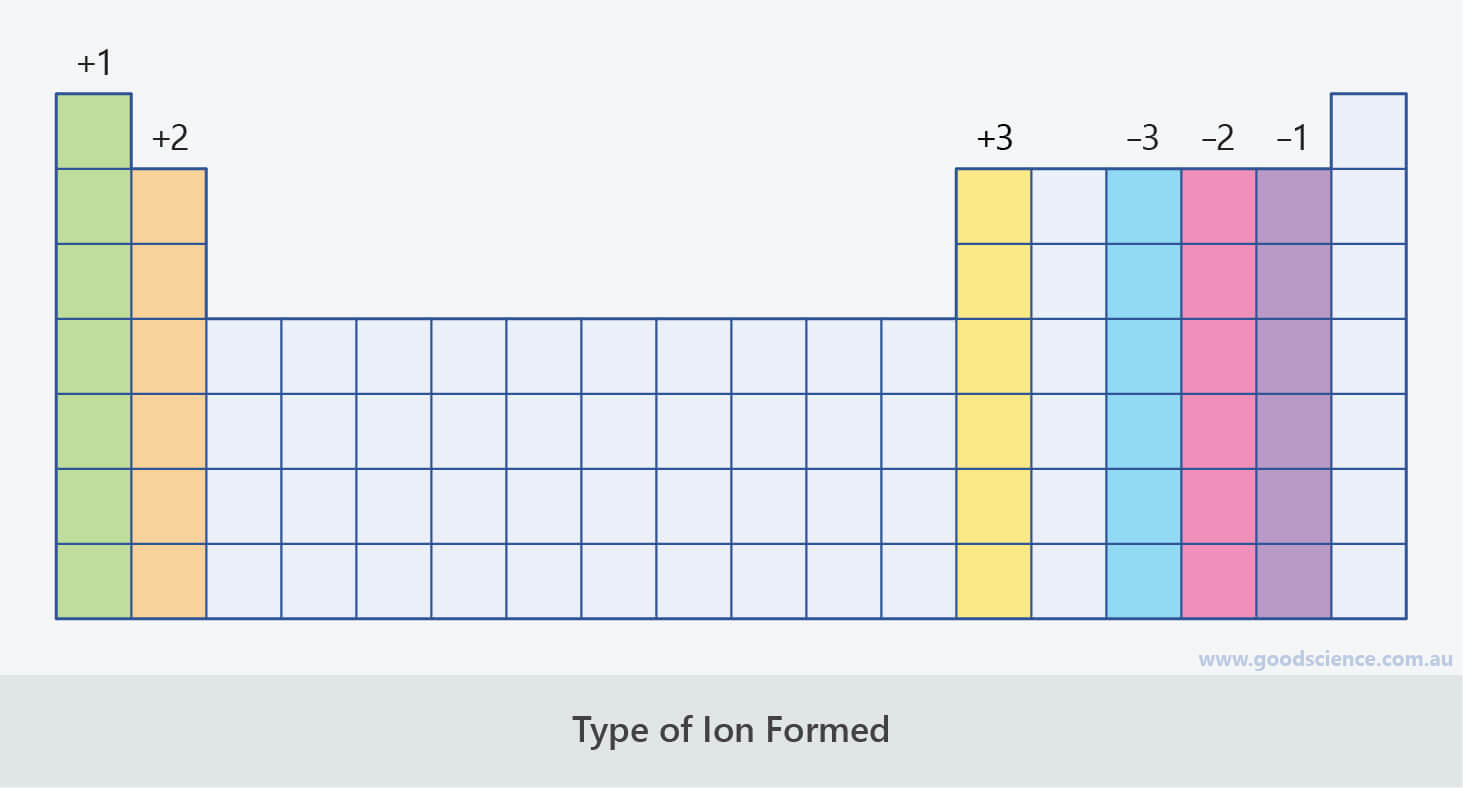
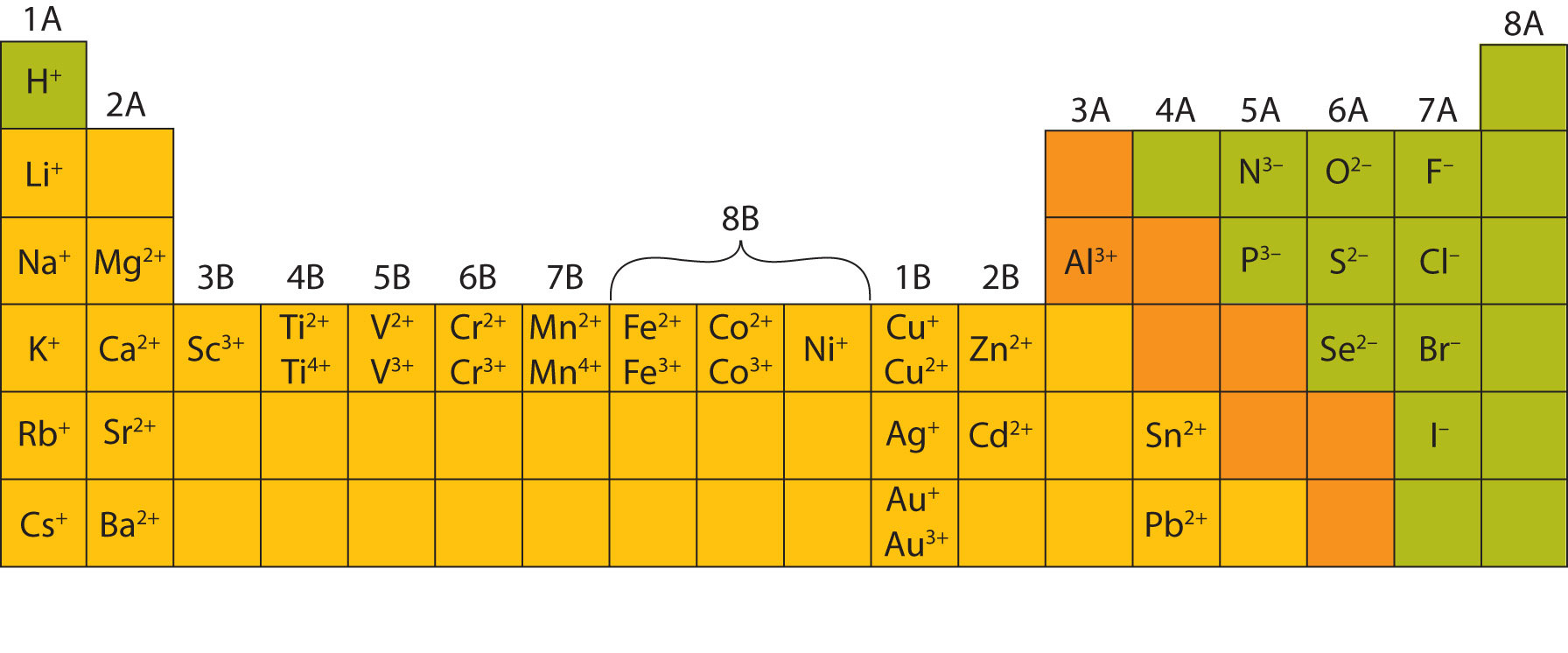
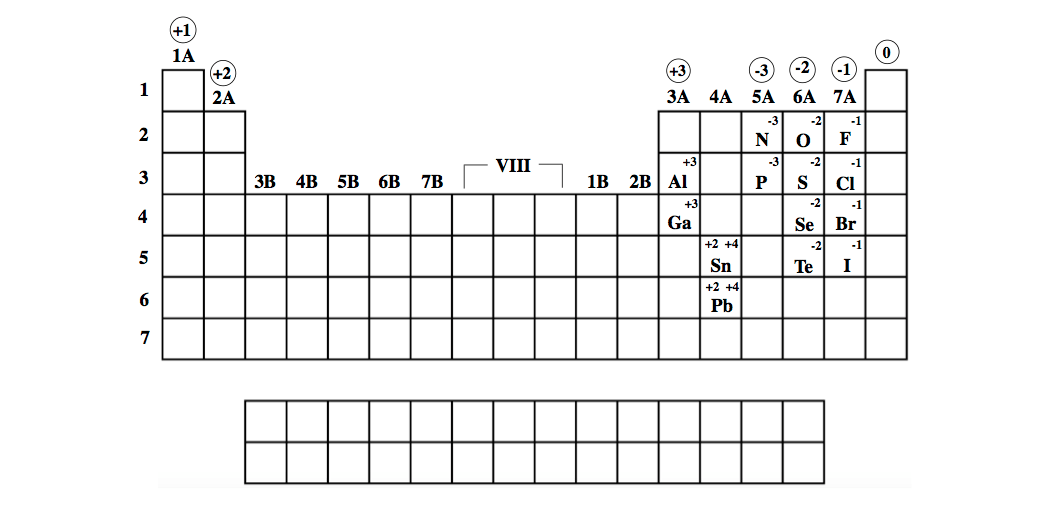
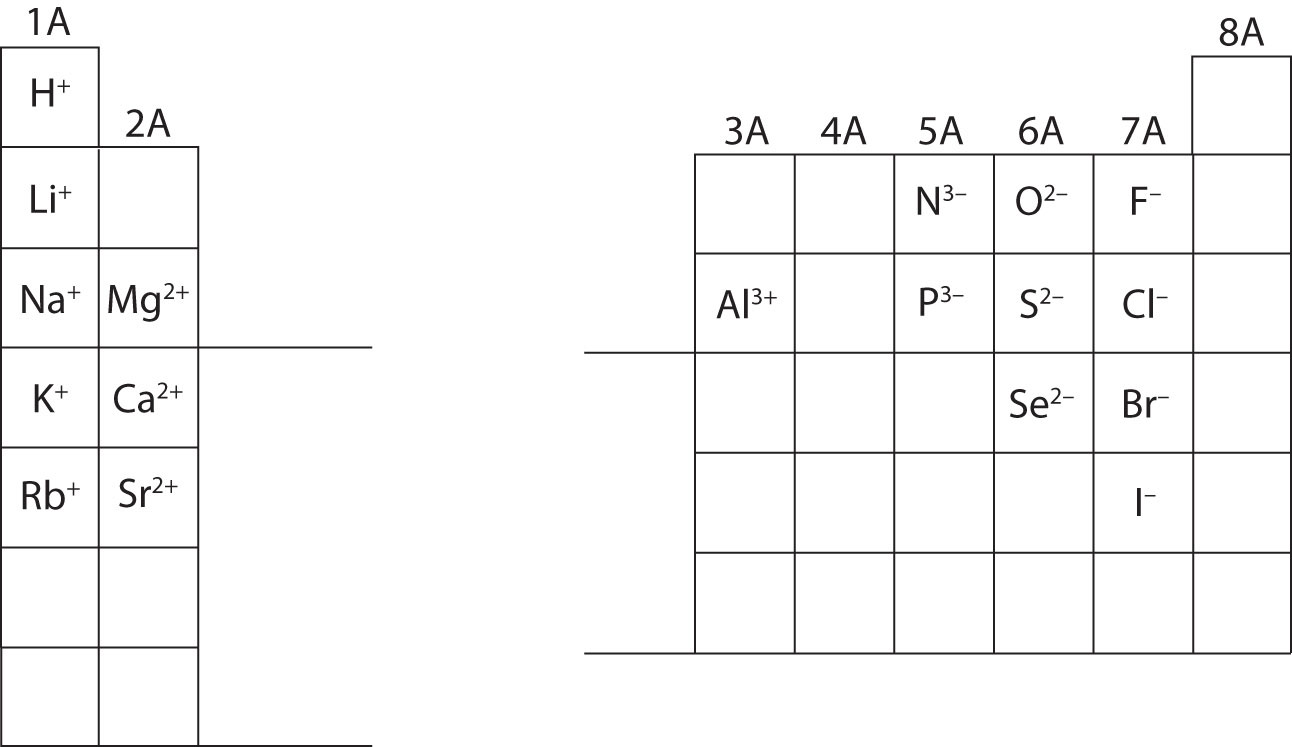
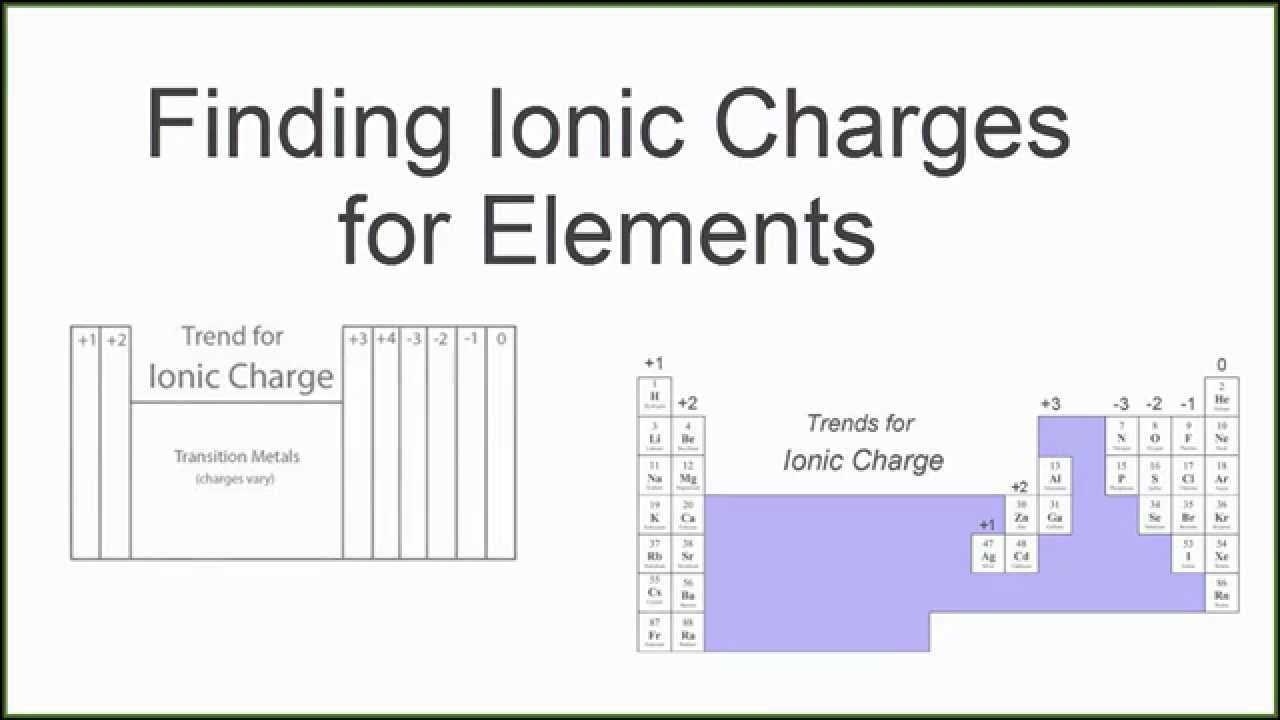
The periodic table ionic charge can be broken down by metals that are positive and on the left of the table and nonmetals which are negative and found on the right.

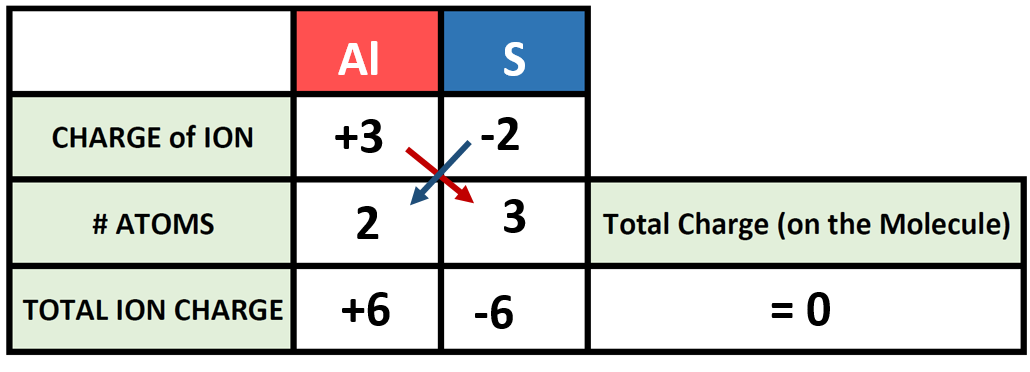
Periodic table charges ions. How to find the ionic charge for an element. Group i alkali metals carry a 1 charge group ii alkaline earths carry a 2 group vii halogens carry 1 and group viii noble gases carry a 0 charge. Likewise the periodic table with charges illustrates elements with the probable charges when they are in the ionic form.
There can be no doubt that any science student who is interested in chemistry must learn the periodic table. Group one is composed of metals that have a 1 charge while all the metals in groups 23456789101112 and 16 have a charge 2. Now you can use periodic table trends to predict the most common element charges.
For example copper usually has a 1 or 2 valence while iron typically has a 2 or 3 oxidation state. Then metals in groups thirteen and fifteen have a charge of 3. This is especially the case when you are writing formulas based on the chemical name.
The periodic table can also be broken down by name and mass depending on your interests. And a periodic table with atomic mass and charges interprets both the charges and atomic mass. The rare earths often carry many different ionic charges.
The elements in a same group tends to form ions with the same charge. If you look at the periodic table you will find the metals in groups from one to 16. Finally all the metals in group 14 have a 4 charge.
It is most consistent at each end of the periodic table. Acetate periodic table of ions arsenate arsenite benzoate borate bromate carbonate chlorate chlorite chromate cyanate cyanide dichromate ch3coo aso4 3 aso3 3 c6h5coo bo3 3 bro3 co3 2 clo3 clo2 cro4 2 cno cn cr2o7 2 oxalate perchlorate periodate permanganate peroxide phosphate pyrophosphate sulfate. When you are working with ionic compounds they are called ionic because they consist of ions youll often need to determine the ionic charge.
/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)










:max_bytes(150000):strip_icc()/PeriodicTableCharge-BW-56a12db13df78cf772682c34.png)









0 Response to "Periodic Table Charges Ions"
Post a Comment