Periodic Table Helium Atomic Number
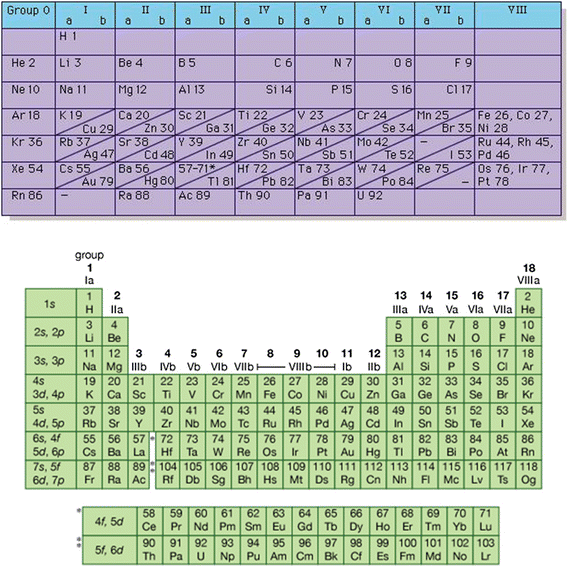
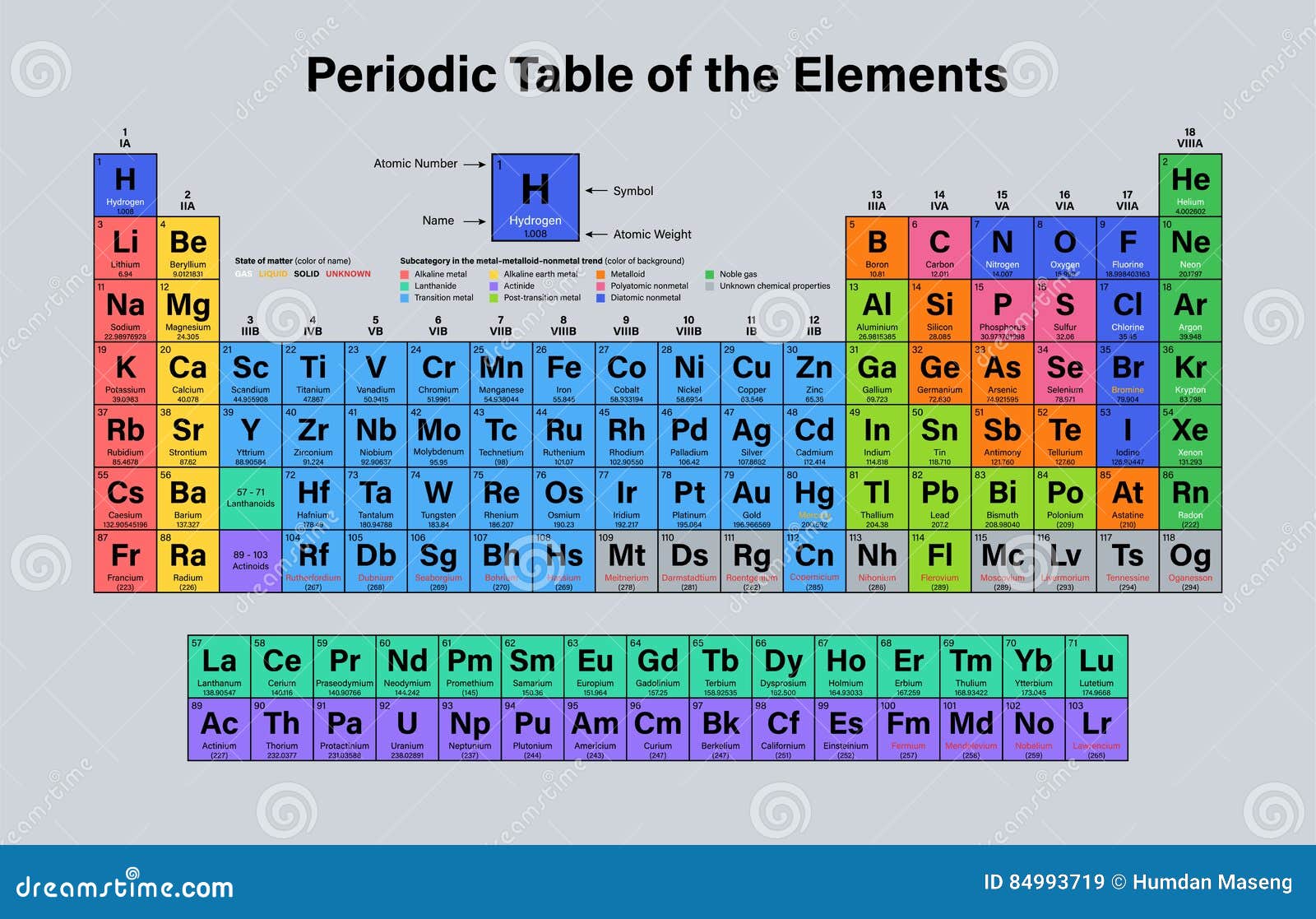
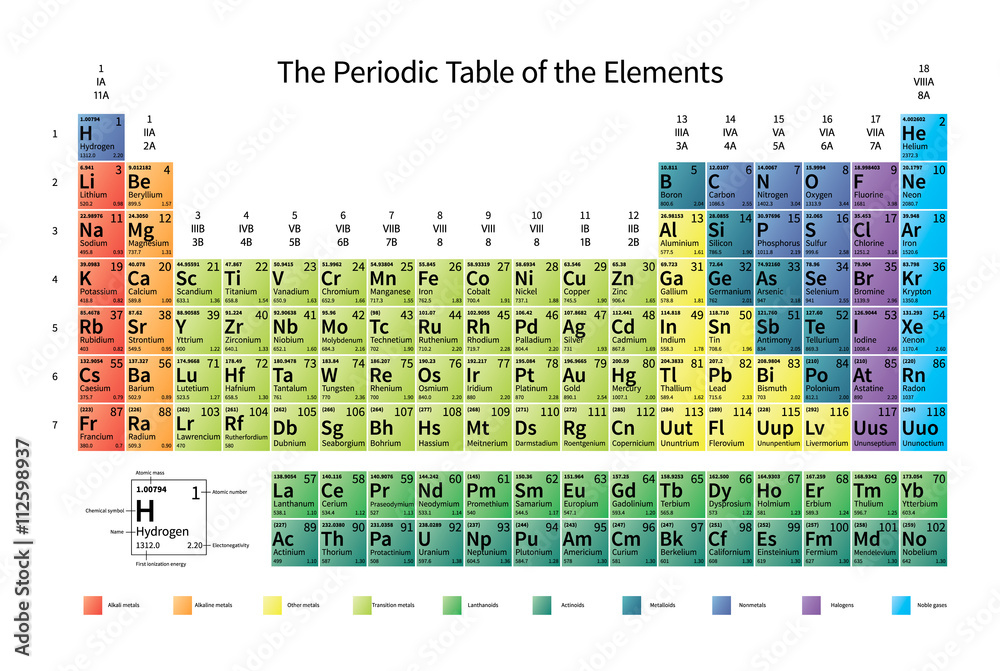
The number of inert gas group is 18 in the modern periodic table. Members of a group typically have similar properties and electron configurations in their outer shell.
Its boiling point is the lowest among all the elements.
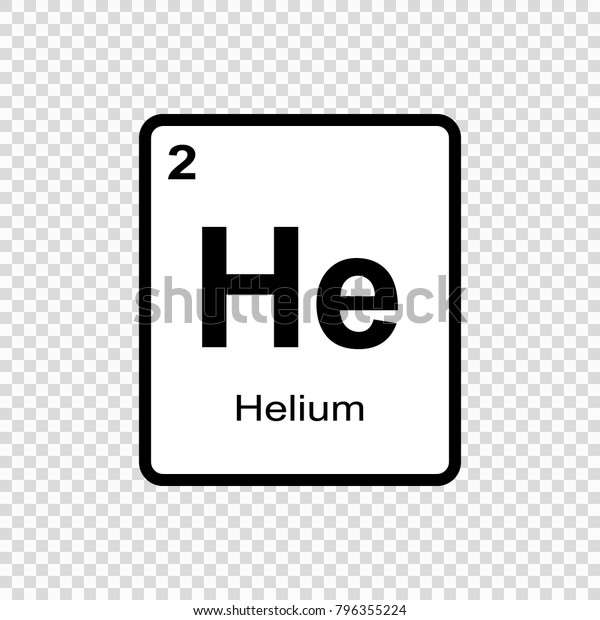
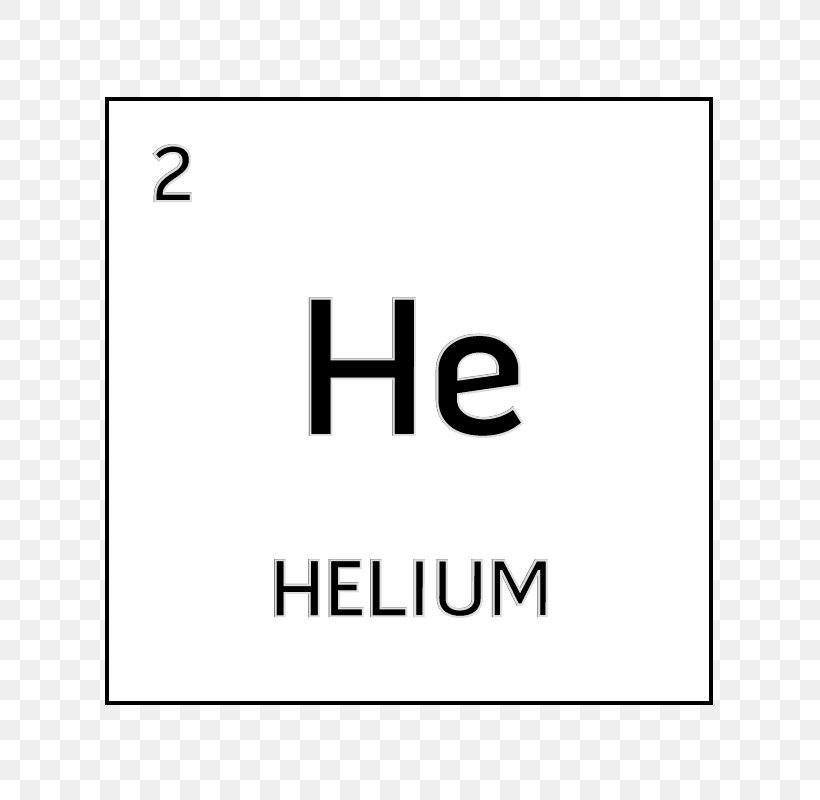
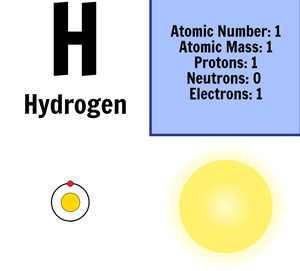
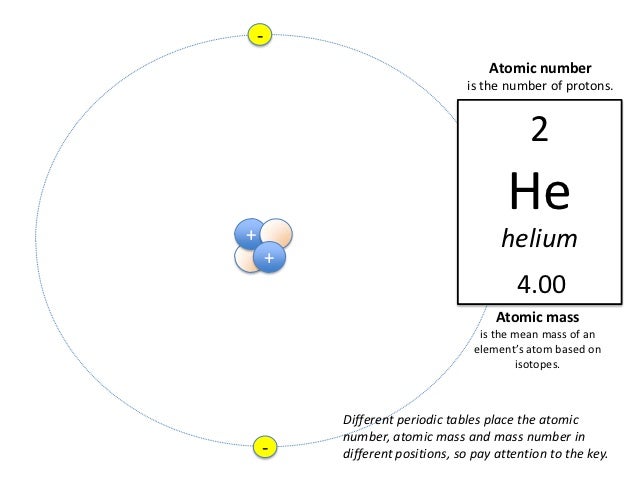
Periodic table helium atomic number. The chemical symbol for helium is he. Atomic number of helium. Number of protons in helium is 2.
It is a colorless odorless tasteless non toxic inert monatomic gas the first in the noble gas group in the periodic table. Sun is a chemical element with the symbol he and atomic number 2. Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure.
A vertical column in the periodic table. Inert gases are also called noble gases. Atomic weight of helium is 4002602 u or gmol.
Since other inert gases have higher atomic number than helium they are placed in the successive lower positions in the inert gases group. The atomic number of each element increases by one reading from left to right. Consequently the smallest atom is helium with a radius of 32 pm while one of the largest is caesium at 225 pm.
Atomic number of helium. Atomic number of helium is 2. Chemical symbol for helium is he.
On the periodic table of the elements atomic radius tends to increase when moving down columns but decrease when moving across rows left to right. The atomic radii decrease across the periodic table because as the atomic number. Melting point of helium is 2722 0c and its the boiling point is 2689 0c.
Period a horizontal row in the periodic table. Hence helium is grouped in the list of inert gases.









:max_bytes(150000):strip_icc()/element-list-names-atomic-numbers-606529_V1_FINAL-f332cfc84a494b7782d84fc986cdaf86.png)





















0 Response to "Periodic Table Helium Atomic Number"
Post a Comment