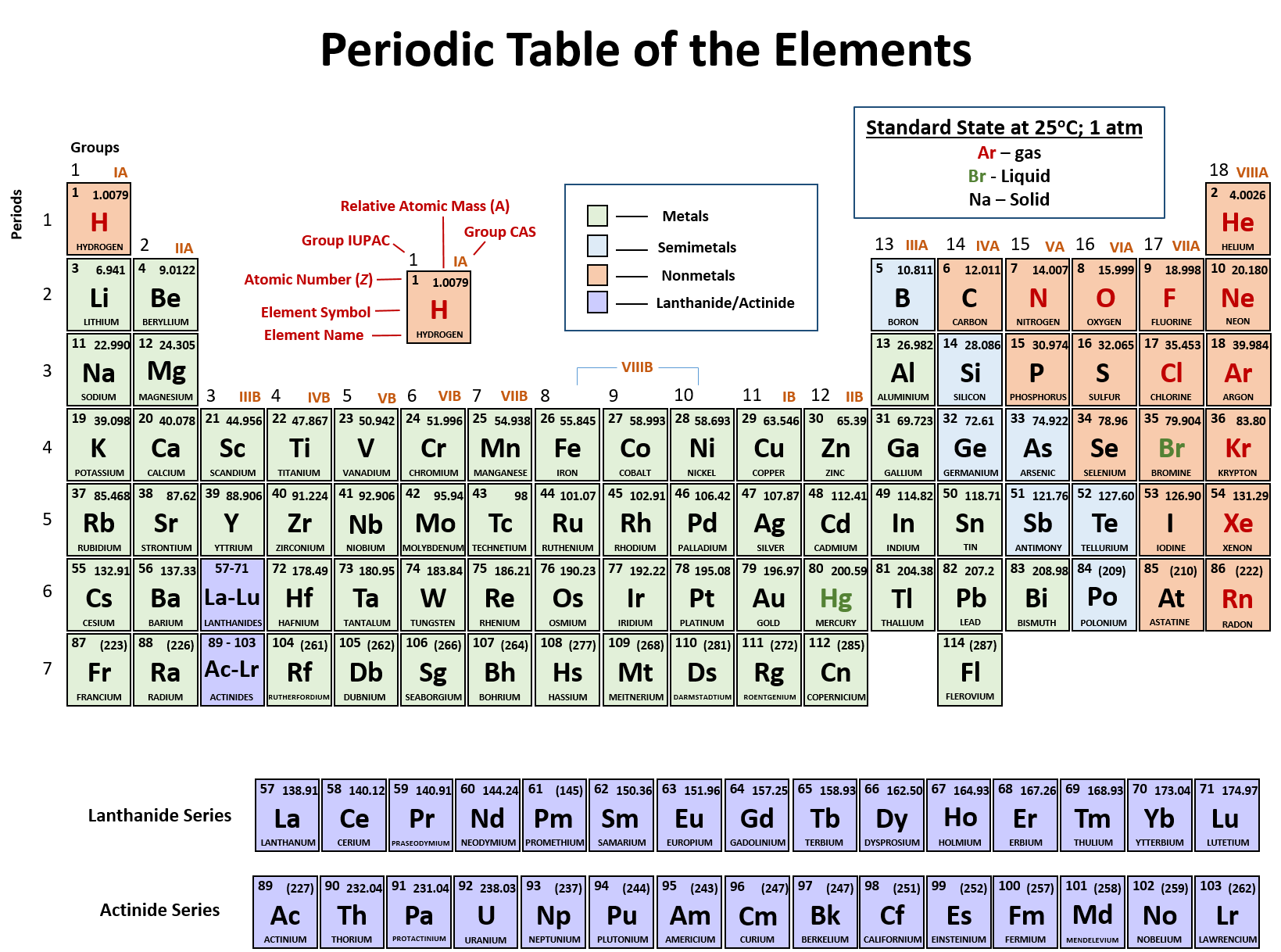
Periodic Table Chemistry Definition
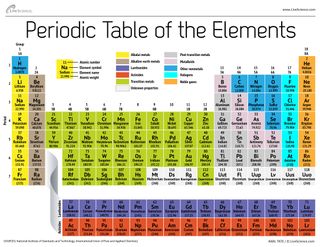
Period 1 contains only two elements. Chemistry the tabular arrangement of the elements according to their atomic numbers so that elements with similar properties are in the same column.
The rows in the periodic table the periods reflect the filling of electrons shells around the nucleus so when a new row begins the elements stack on top of each other with similar properties.
Periodic table chemistry definition. Elements having similar. The periodic table is a tabular arrangement of chemical elements that is arranged by increasing atomic number and groups elements according to recurring properties. Elements within a group column display similar characteristics.
Periodic table periodic table n. Award winning periodic table with user friendly element data and facts. Periodic table perdk n chemistry a table of the elements arranged in order of increasing atomic number based on the periodic law.
A period in the periodic table is a row of chemical elementsall elements in a row have the same number of electron shellseach next element in a period has one more proton and is less metallic than its predecessor. Cool online chemistry videos dictionary tools etc. Apart from period 1 every period begins with an alkali metal and ends with a noble gas.
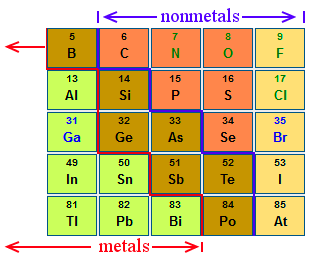
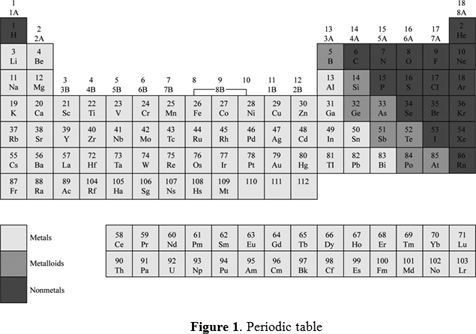
The rows are arranged so that metals are on the left side of the table and nonmetals are on the right side. Arranged this way groups of elements in the same column have similar chemical and physical properties reflecting the periodic lawfor example the halogens lie in the second last. Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number.
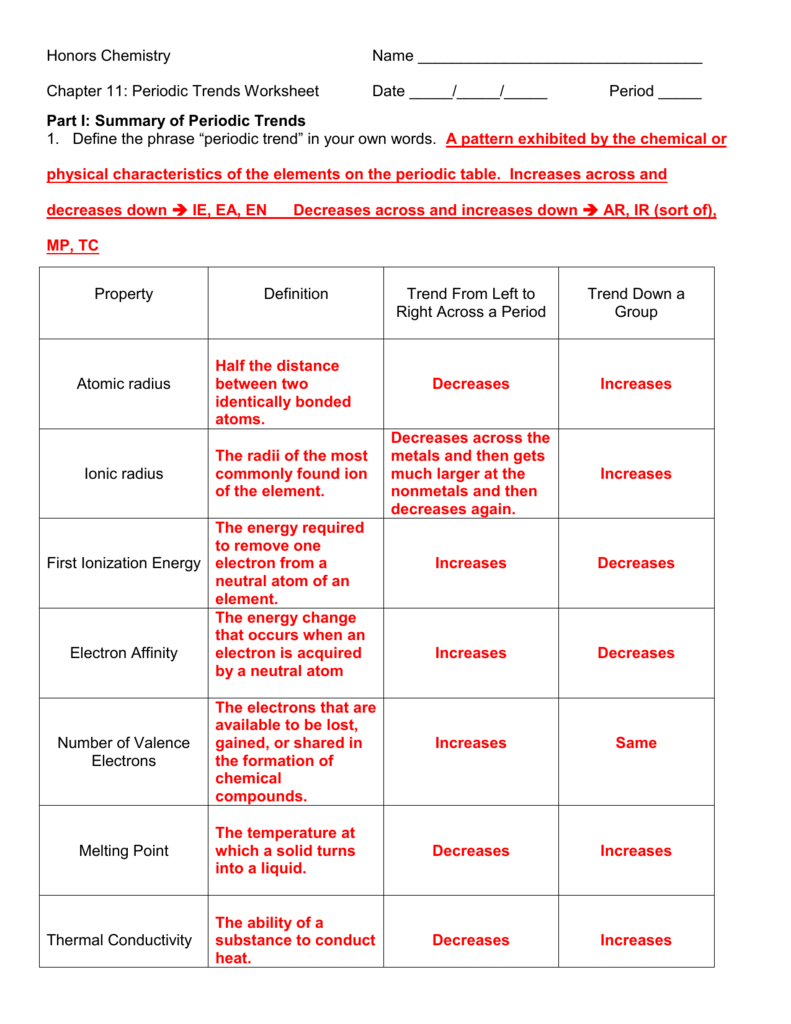
The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical properties. When the elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. The structure of the table shows periodic trends.
Mendeleev organized elements according to recurring properties to make a periodic table of elements. The periodic table has seven periods. Periodic table definition a table illustrating the periodic system in which the chemical elements formerly arranged in the order of their atomic weights and now according to their atomic numbers are shown in related groups.
The seven rows of the periodic table are called periods. Hydrogen and helium. Interactive periodic table of elements your complete guide to the elements including definition mass names of each chemical in the periodic table.




/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)




/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)




:max_bytes(150000):strip_icc()/periodic-table-165930186-590f2d703df78c92832fe141.jpg)






:max_bytes(150000):strip_icc()/accurate-illustration-of-the-periodic-table-82020791-57cc76f23df78c71b66efbd7.jpg)




0 Response to "Periodic Table Chemistry Definition"
Post a Comment