Periodic Table With Groups And Valence Electrons
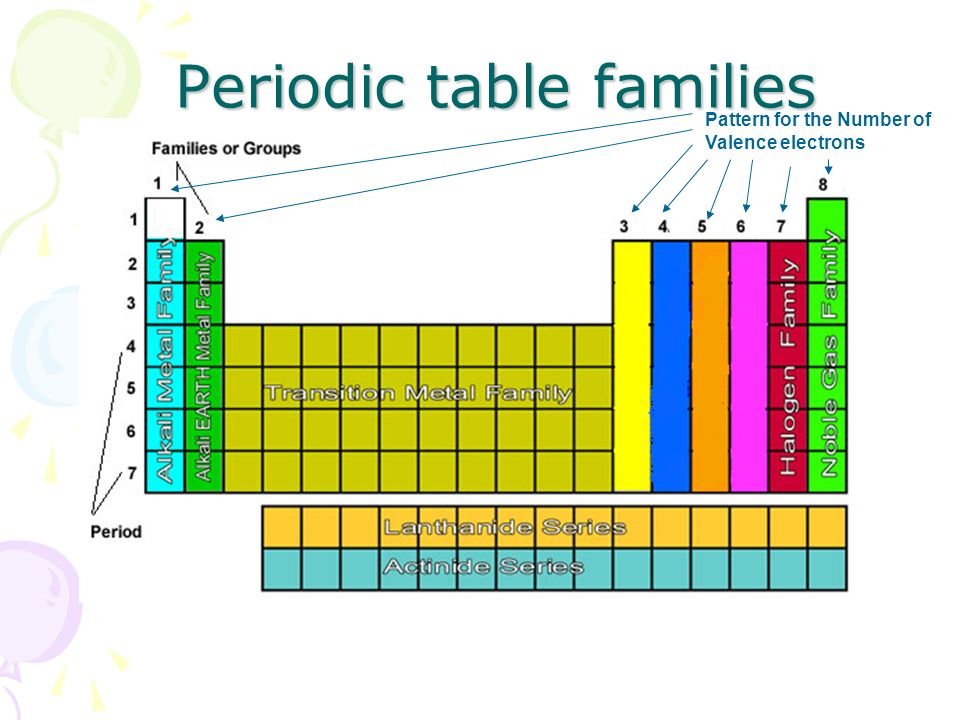
1 through 18 are called groups. Chemistry periodic table basics chemical laws molecules projects experiments scientific method.
Periodic table of element groups.

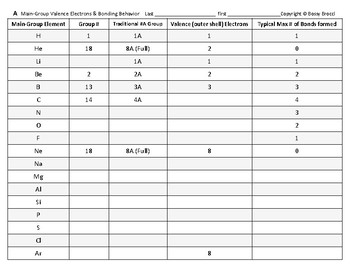
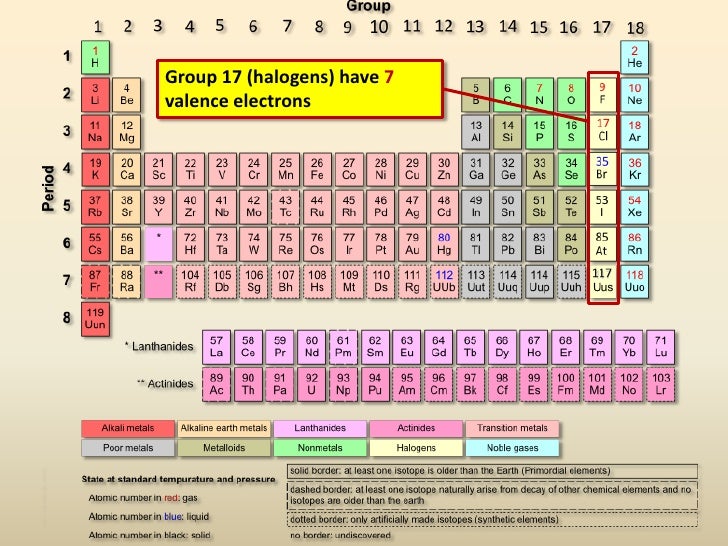
Periodic table with groups and valence electrons. Seven valence electrons so elements from this group typically exhibit a 1 oxidation state. How do an elements valence electrons relate to its group in the periodic table. In the periodic table elements with similar chemical properties are in the same group.
Valence electrons are the electrons present in the outermost shell of an atom. Organization gets a bit murky with the group b. This printable periodic table contains the most common valence charges of the elements.
Periods correspond to the number of electron shells possessed by atoms of the elements in that row. Search the site go. The noble gasses have complete valence electron shells so they act differently.
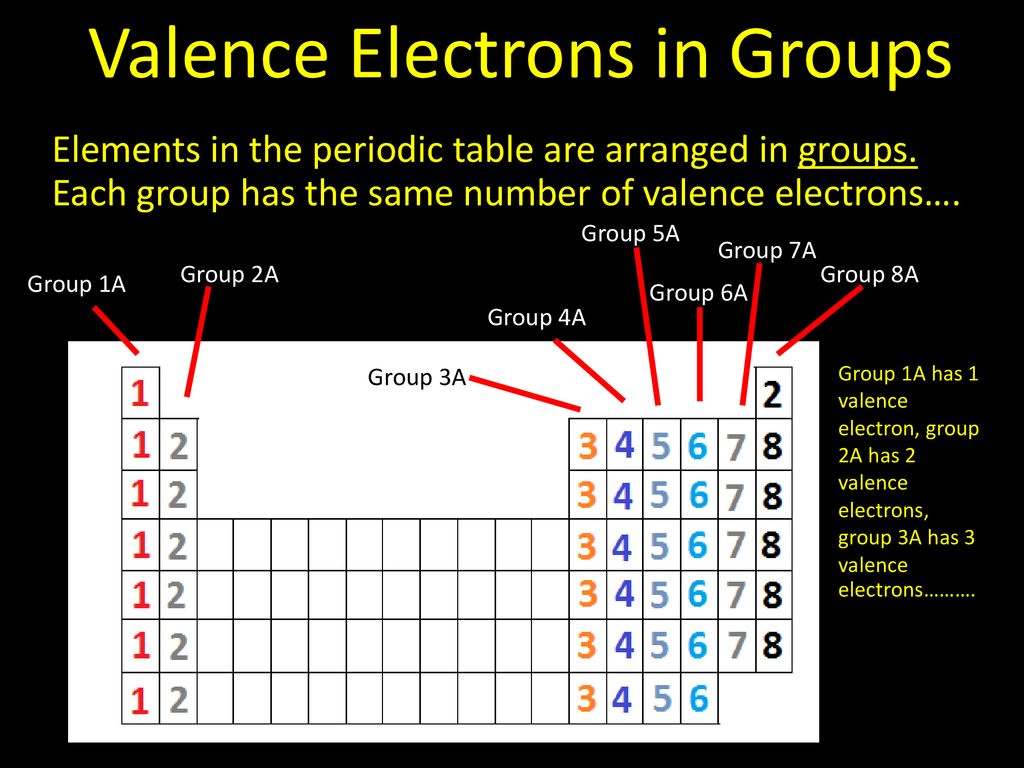
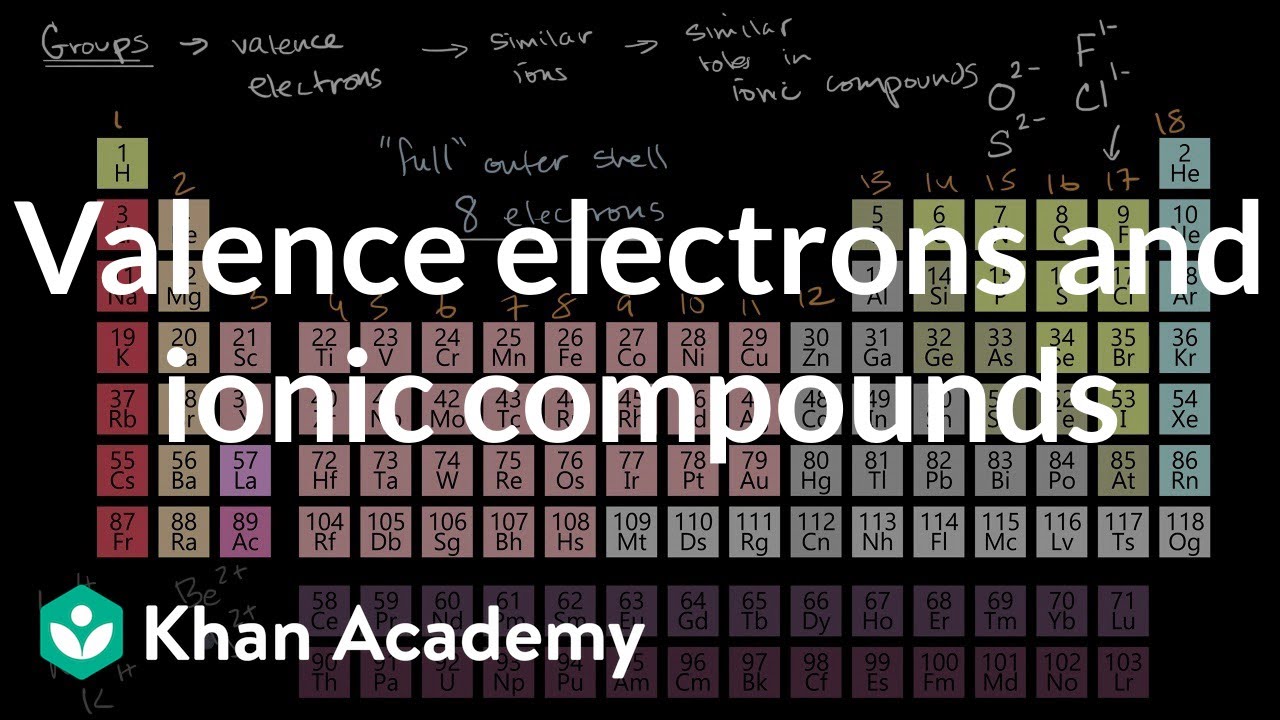
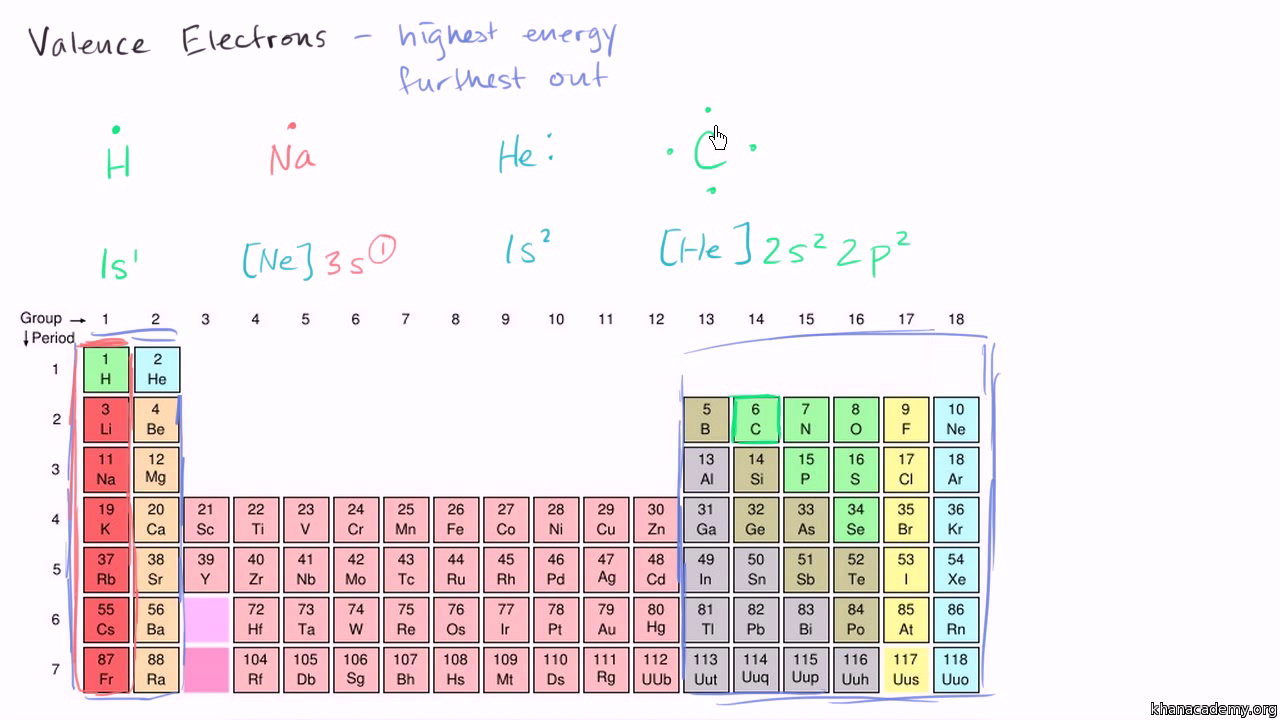
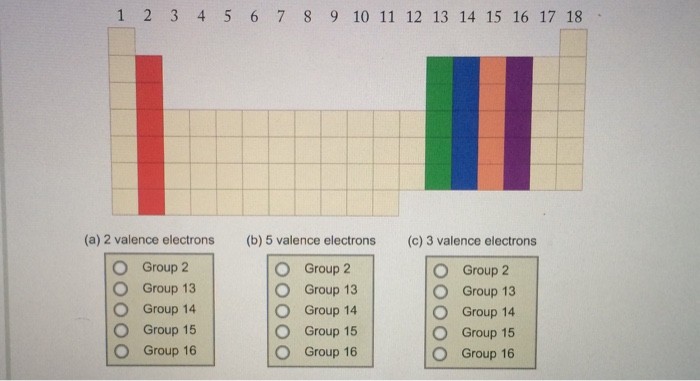
Elements in group 1a have only one valence electron and each group a column to the right adds one more valence electron. For example atoms in groups 1 and 2 have 1 and 2 valence electrons respectively. Now that weve classified our elements into groups on the periodic table lets see how to determine the number of valence electrons.
Learn vocabulary terms and more with flashcards games and other study tools. You can easily determine the number of valence electrons an atom can have by looking at its group in the periodic table. This printable periodic table contains the most common valence charges of the elements.
In chemistry the valence or valency of an element is a measure of its combining power with other atoms when it forms chemical compounds or moleculesthe concept of valence was developed in the second half of the 19th century and helped successfully explain the molecular structure of inorganic and organic compounds. Unlike other groups noble gasses are unreactive and have very low electronegativity or electron affinity. Printable periodic table of the elements with valence charges.
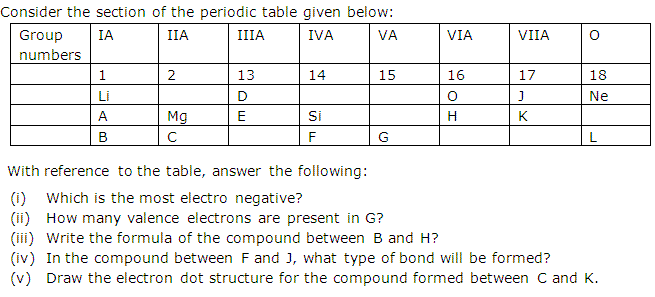
Start studying periodic table group names and number of valence electrons. You may assume that the valences of the elementsthe number of electrons with which an atom will bond or formare those that can be derived by looking at the groups columns of the periodic table. While these are the most common valences the real behavior of electrons is less simple.
With the exception of groups 312 the transition metals the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column. Oxygen is found in period 2 group 16. The quest for the underlying causes of valence led to the modern theories of.
And so for this video were only talking about the valence electrons for elements in the main groups. The number of valence electrons of an element can be determined by the periodic table group vertical column in which the element is categorized. Set up to group elements by similar chemical properties turn out to be the exact same columns defined by the number of valence electrons.
The horizontal rows of the periodic table from 1 to 7 are called periods. The number of valence electrons. How to figure valence of electrons in the periodic table.
























0 Response to "Periodic Table With Groups And Valence Electrons"
Post a Comment