Radius Size Trend Periodic Table
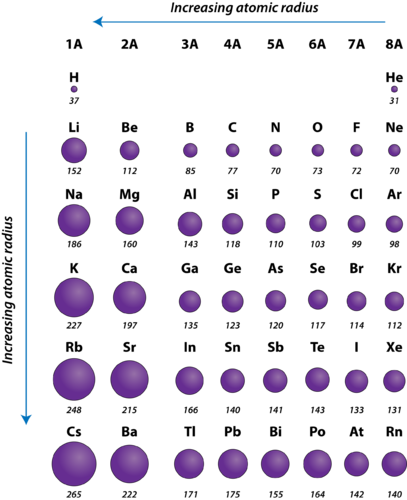
There are other trends to be found in the periodic table apart from the atomic radius trend. If you look at the table you can see there is a clear trend in atomic radius.
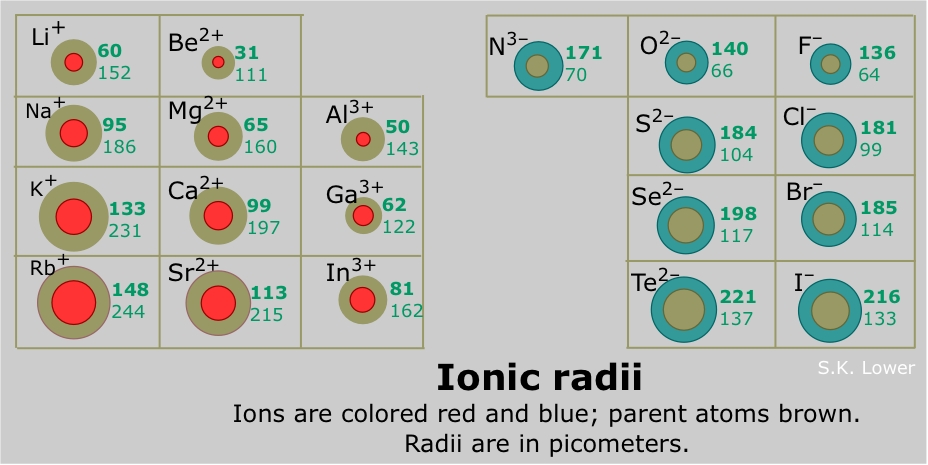
As you move down a column or group the ionic radius increases.

Radius size trend periodic table. In general the trend of the ionic radius has a similar trend to the atomic radius increasing in size as you move across the periodic table. Atomic radius 133. Common periodic trends include those in ionization energy atomic radius and electron affinity.
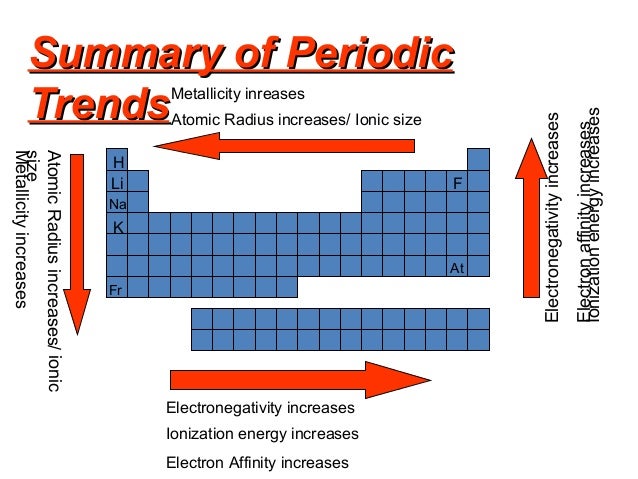
This is because each row adds a new electron shell. Across a period. The following trend in periodic properties of elements is observed.
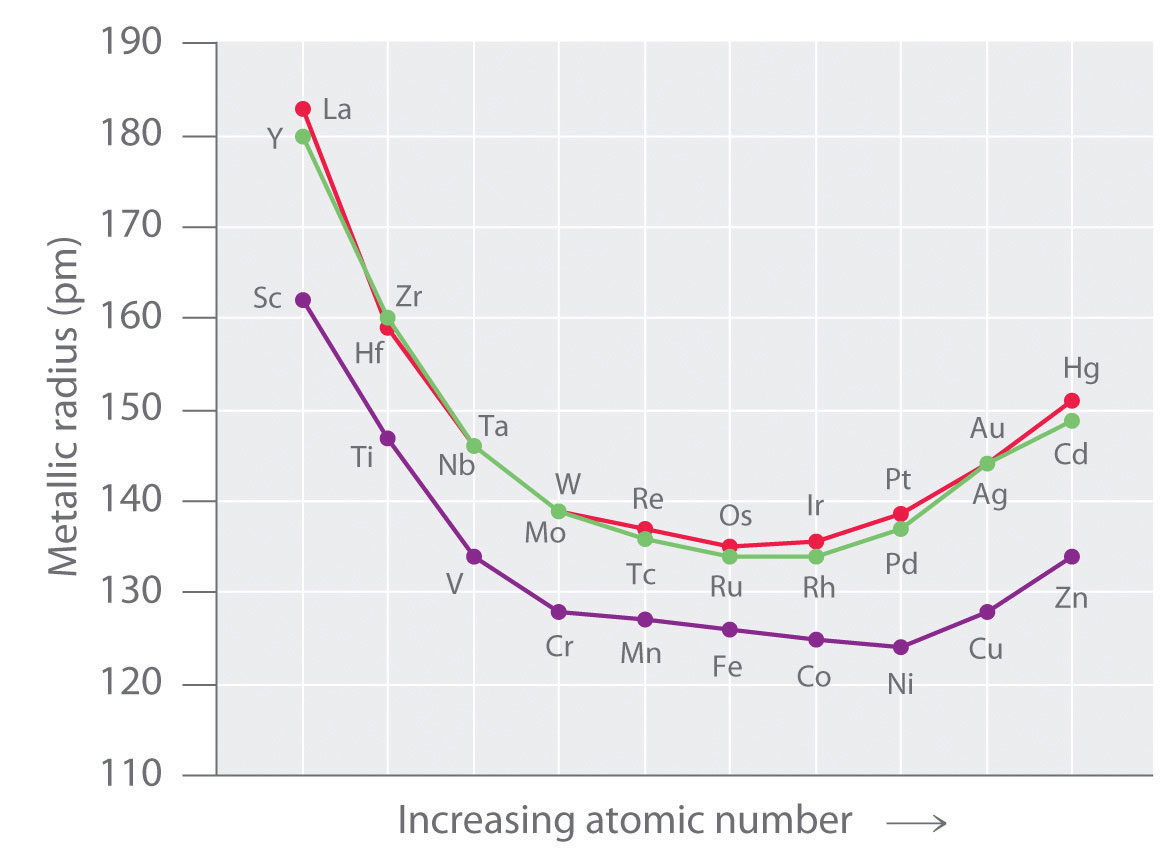
The metallic radius can also be calculated by measuring the distance between the two nuclei of the two atoms in metal cluster. The atomic radius of the different elements tends to increase as you move down the periodic table much as the ionic radius does. When a neutral atom gains or loses an electron creating an anion or cation the atoms radius increases or decreases respectively.
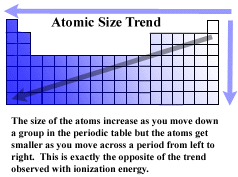
One such trend is closely linked to atomic radii ionic radii. This data helps us understand why some molecules fit together and why other molecules have parts that get too crowded under certain conditions. The general trend of atomic radius is that it increases as you move down a group and decreases as you move to the right across a period.
The size of an elements ionic radius follows a predictable trend on the periodic table. Other trends found in the periodic table. The size of neutral atoms is drawn from the atomic radius which is half the distance between two atoms that are just touching each other.
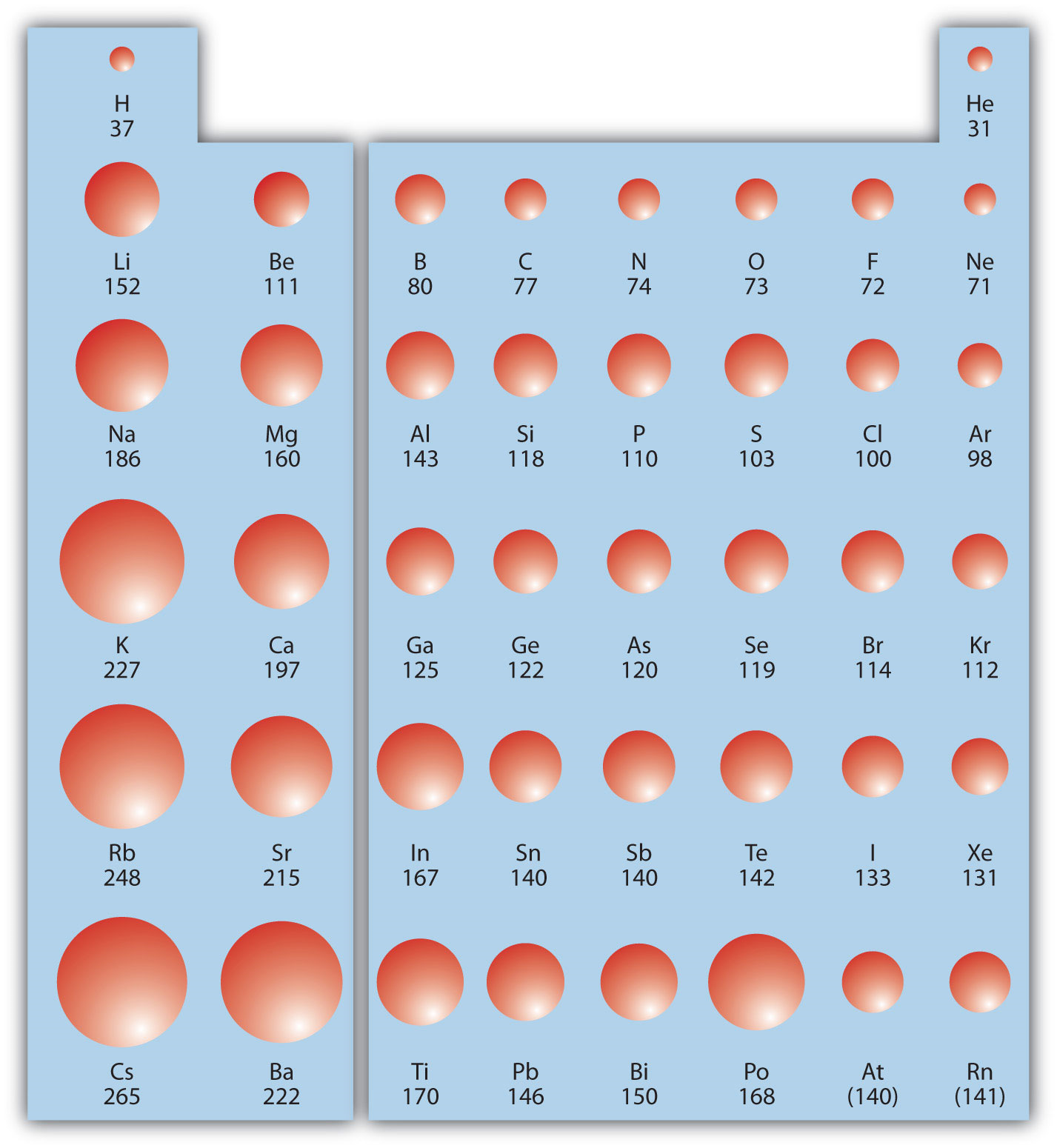
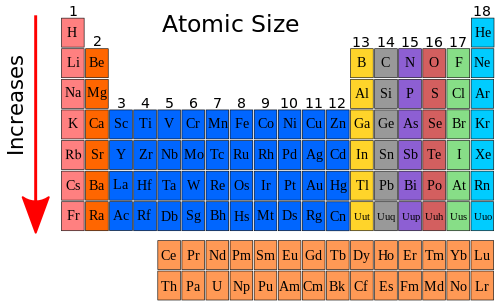
The distance between the center of the nucleus and the outermost shell of an atom is known as the atomic radius. The atomic radius trend reflects the change in the atomic radius that occurs as you follow the periodic table from top to bottom. The atomic radius in the periodic table decreases across the period and increases down the group.
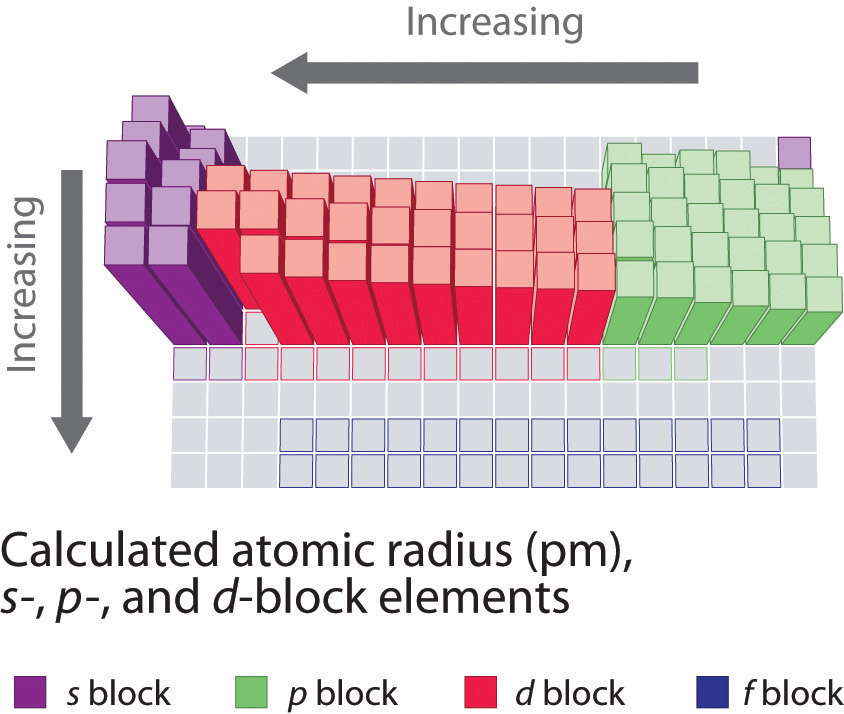
Exceptions are observed in transition metal. Neutral atoms tend to increase in size down a group and decrease across a period. Other trends in the periodic table.
One of the ways we can express the size of atoms is with the atomic radius. In a group the atomic size increases due to the addition of shells as we move from one period to another. Atomic radius is one of the periodic properties of the elements.
Below is a periodic table with arrows showing how atomic radii change to help you understand and visualize each atomic radius trend. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Ionic radius are calculated by considering the atomic size of the two atoms.
The size of atoms is important when trying to explain the behavior of atoms or compounds. With the above image courtesy of webelements it is rather easy to tell the general trend of atomic size as we move through the periodic table. One atomic radius trend occurs as you move left to right across the periodic table moving within a period and the other trend occurs when you move from the top of the periodic table down moving within a group.










/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)


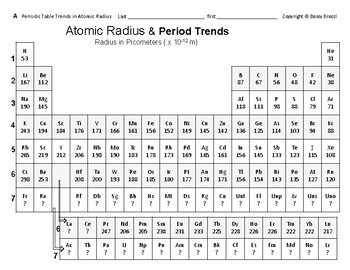



:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)

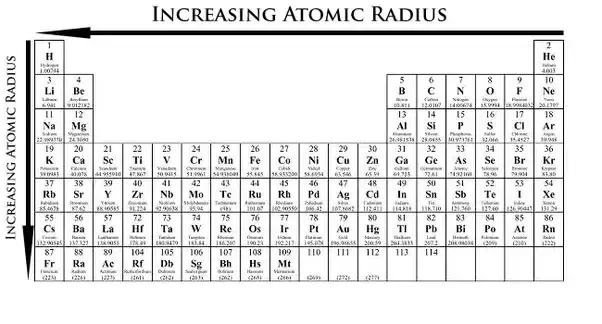




0 Response to "Radius Size Trend Periodic Table"
Post a Comment