Periodic Table Rows And Groups
The periodic table of elements is a large table in which each and every known chemical element is placed in a specific location considering the. Find out in this video from the properties of matter chapter of the virtual.
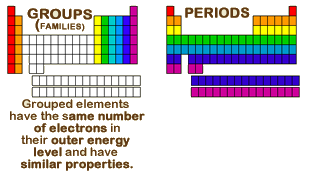
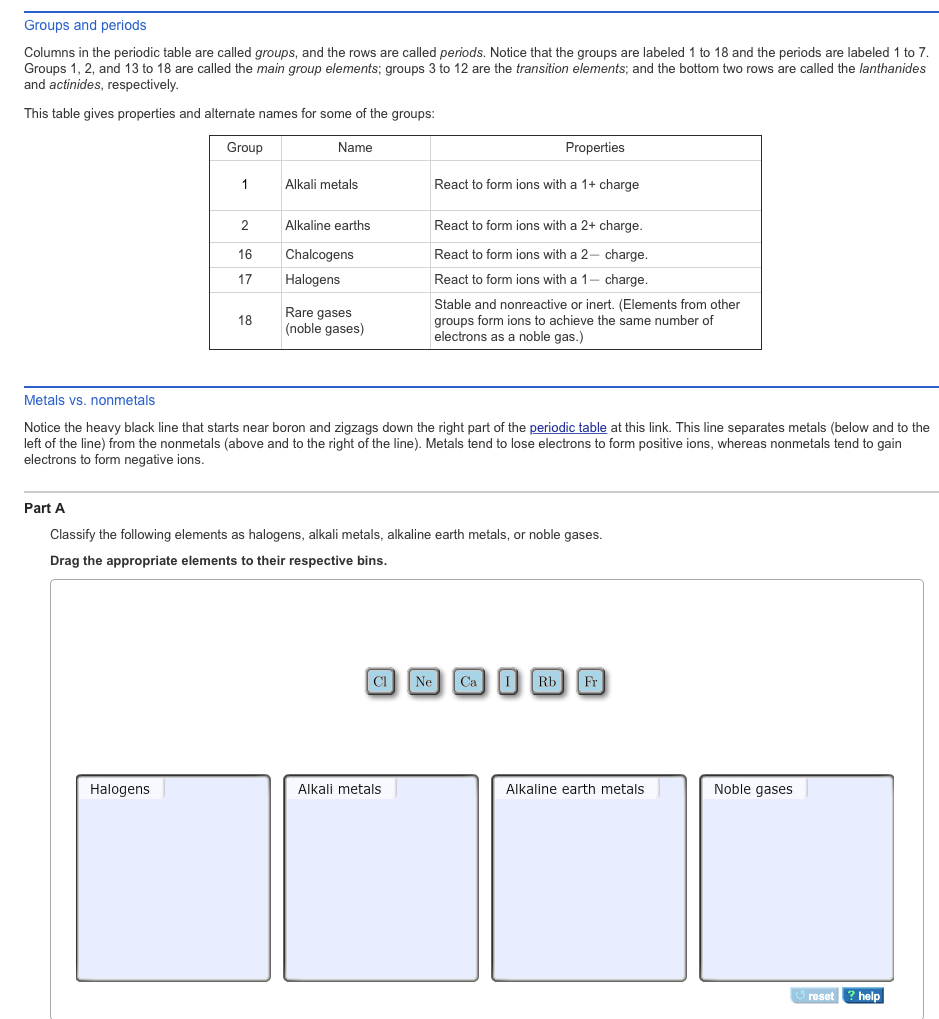
Groups and periods are two ways of categorizing elements in the periodic table.

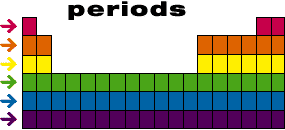
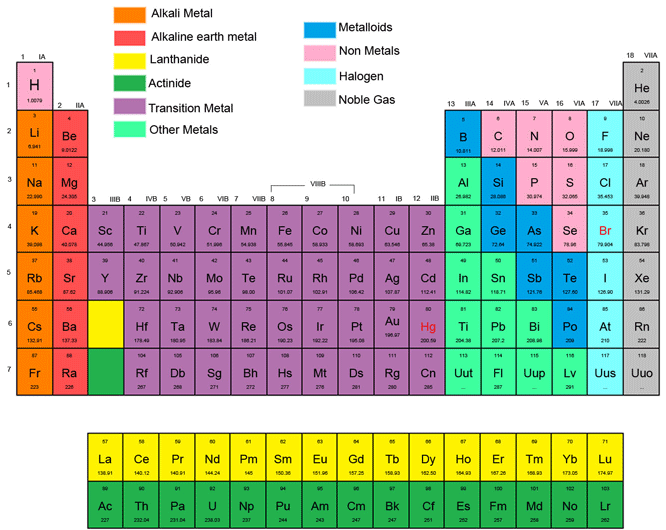
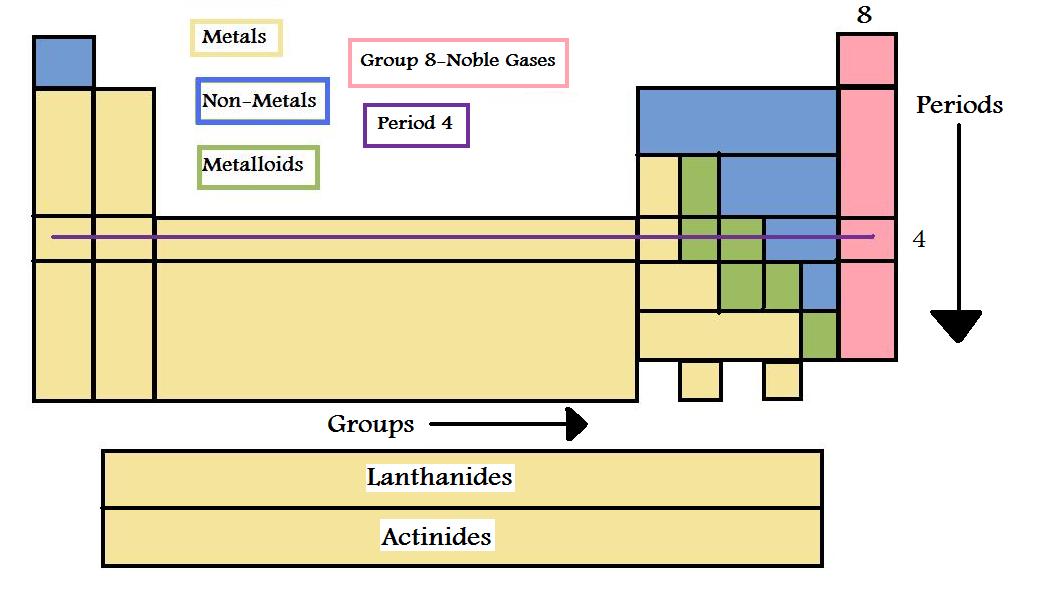
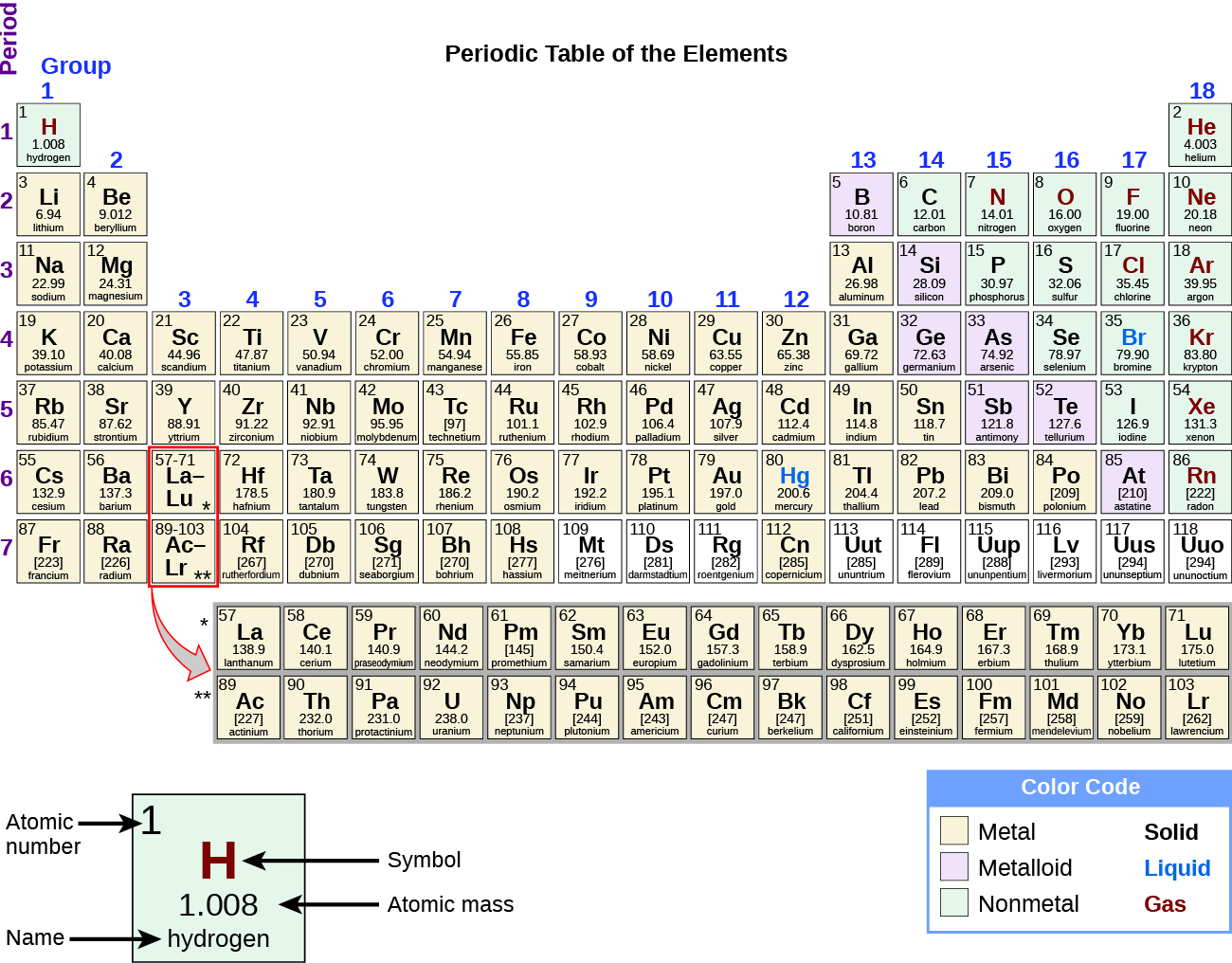
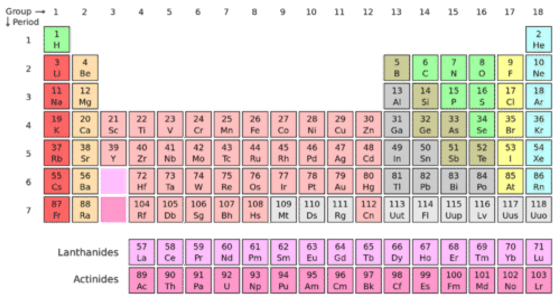
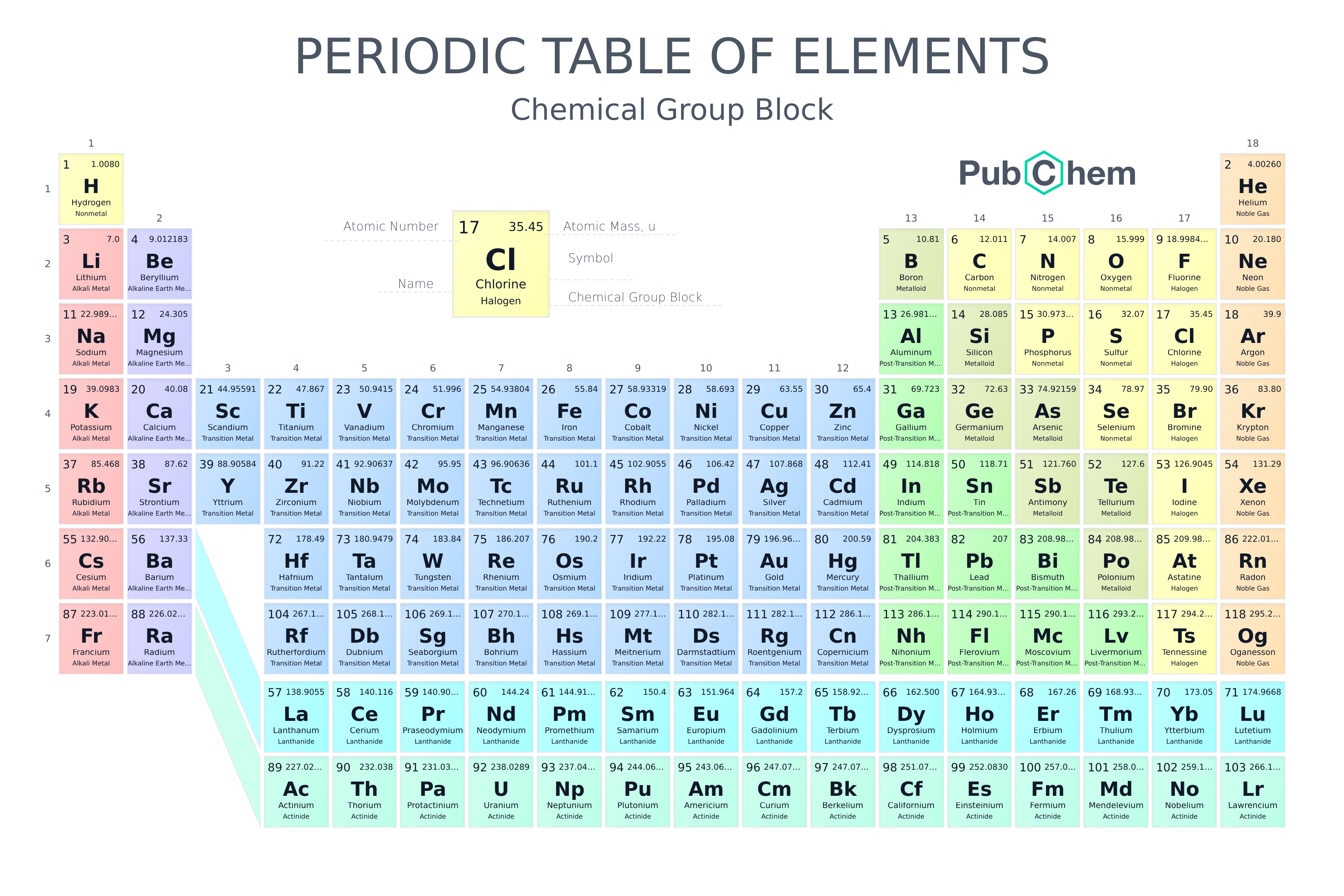
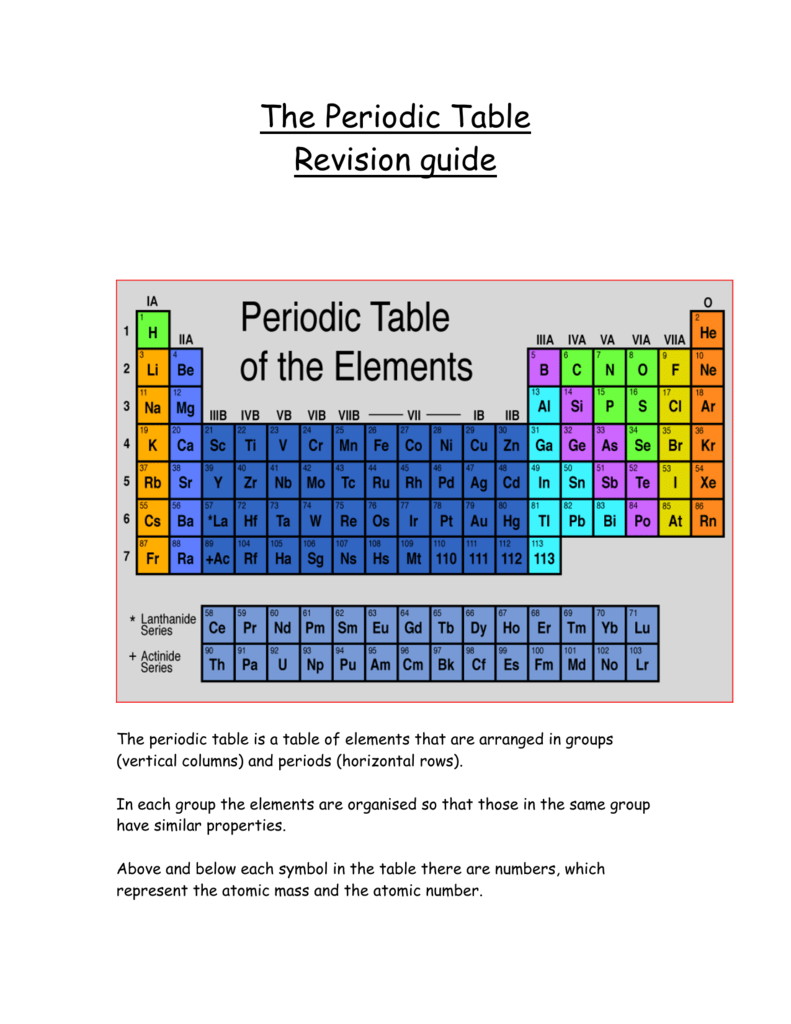
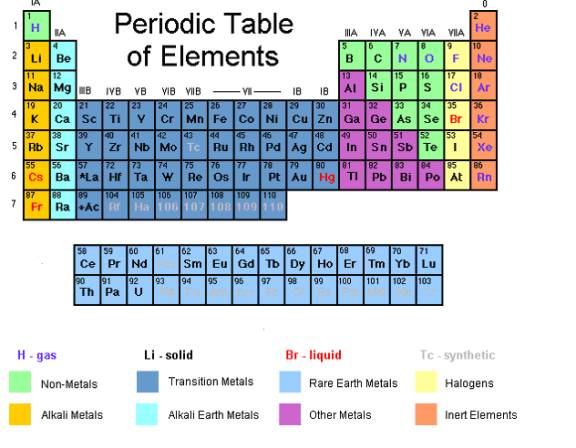
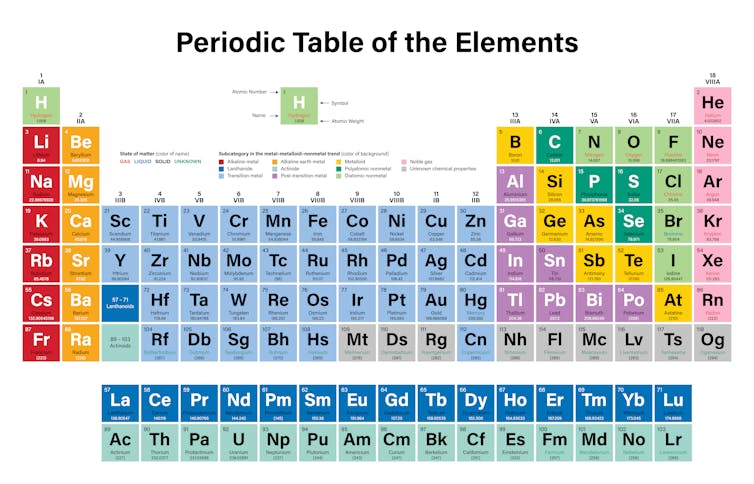
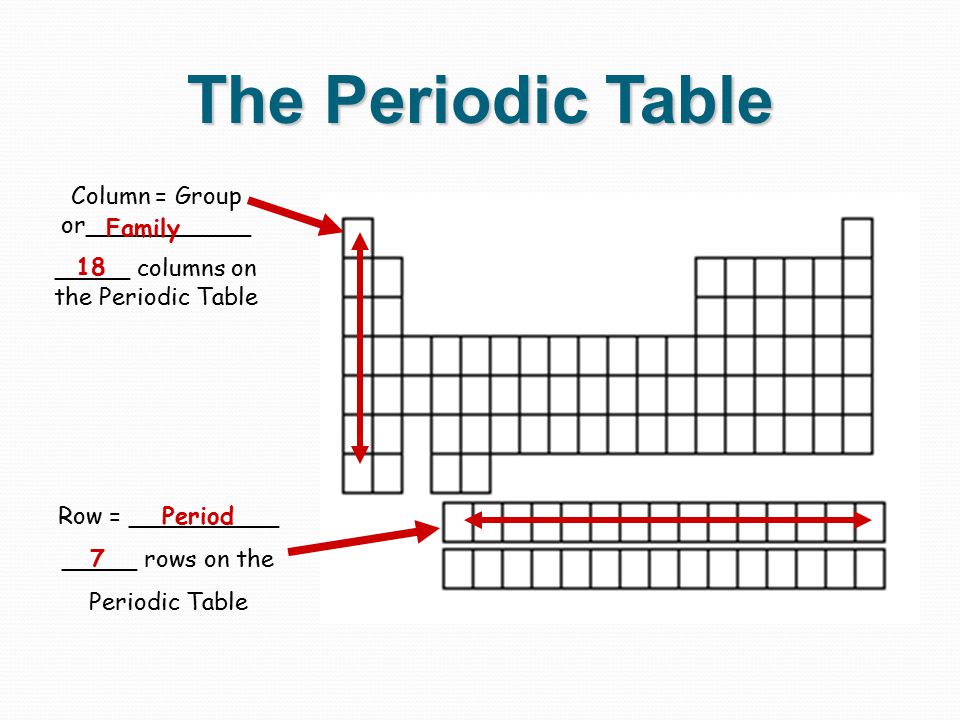
Periodic table rows and groups. The rows are called periods and the columns are called groups. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesthe structure of the table shows periodic trendsthe seven rows of the table called periods generally have metals on the left and nonmetals on the right. The vertical columns of elements are called groups or families.
Hydrogen h is the first element in the periodic table because it has just one proton in its nucleus. When the shell is full a new row is started and the process repeats. Atomic number increases as you move down a group or across a period.
The periodic table is a way of arranging the elements so patterns in their properties and reactions can be identified and explained. Periods in the periodic table in each period horizontal row the atomic numbers increase from left. There are a.
The nonmetals are located on the upper right side of the periodic table separated from metals by a line that cuts diagonally through the periodic table. The number of valence electrons increases from left to right in the period. Periods 6 and 7 have 32 elements because the two bottom rows that are separated from the rest of the table belong to those periods.
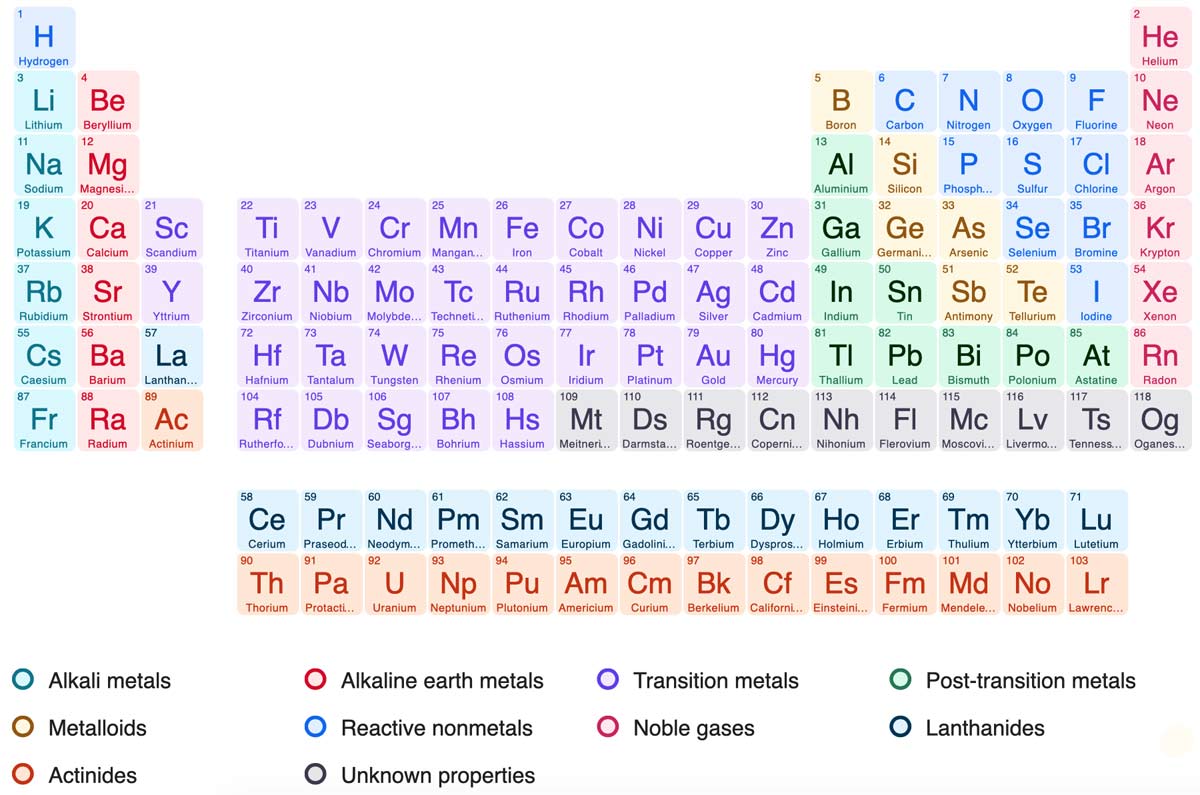
The most common way the periodic table is classified by metals nonmetals and metalloids. The rows on the periodic table are called periods. The halogens and the noble gases are two groups of nonmetals.
Most nonmetals gain electrons easily. In the periodic table of elements there are seven horizontal rows of elements called periods. Periods are horizontal rows across the periodic table while groups are vertical columns down the table.
Whats the difference between periods and groups in the periodic table and why are the elements structured this way. The key difference between periods and groups is that the periods are horizontal rows whereas the groups are the vertical columns in the periodic table of chemical elementsthere are 7 major periods and 18 groups in the periodic table of elements. They are pulled out in order to make the table itself fit more easily onto a single page.
All the elements in a period have valence electrons in the same shell. The nonmetals can be divided into classes of elements that have similar properties. As with any grid the periodic table has rows running left to right and columns running up and down.
Most elements are metals. A group is a vertical column of the periodic table based on the organization of the outer shell electrons.















:max_bytes(150000):strip_icc()/GettyImages-470784875-e7ea7f0da3d74a639d8e6a290afa000b.jpg)


0 Response to "Periodic Table Rows And Groups"
Post a Comment