Group Family Periodic Table Definition
The explanation of the pattern of the table is that the elements in a group have similar physical or chemical characteristic of the outermost electron shells of their atoms ie. Columns of the periodic table typically mark groups or families.
Three systems have been used to number families and groups.

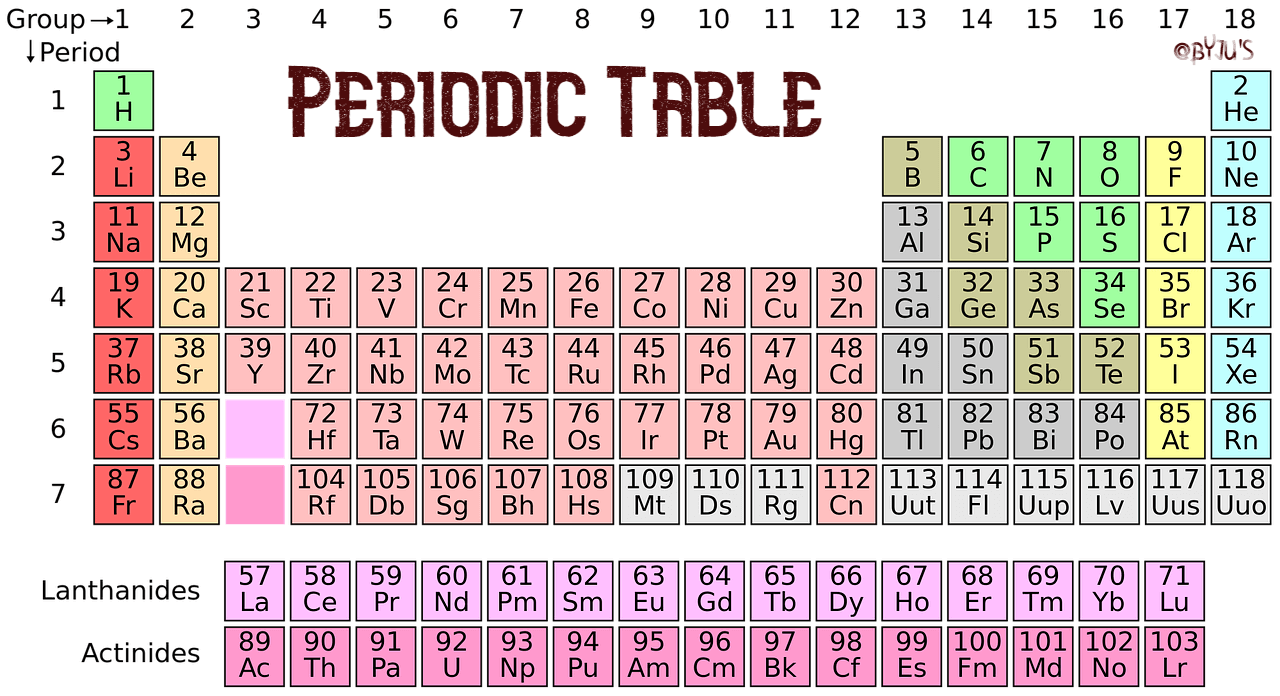
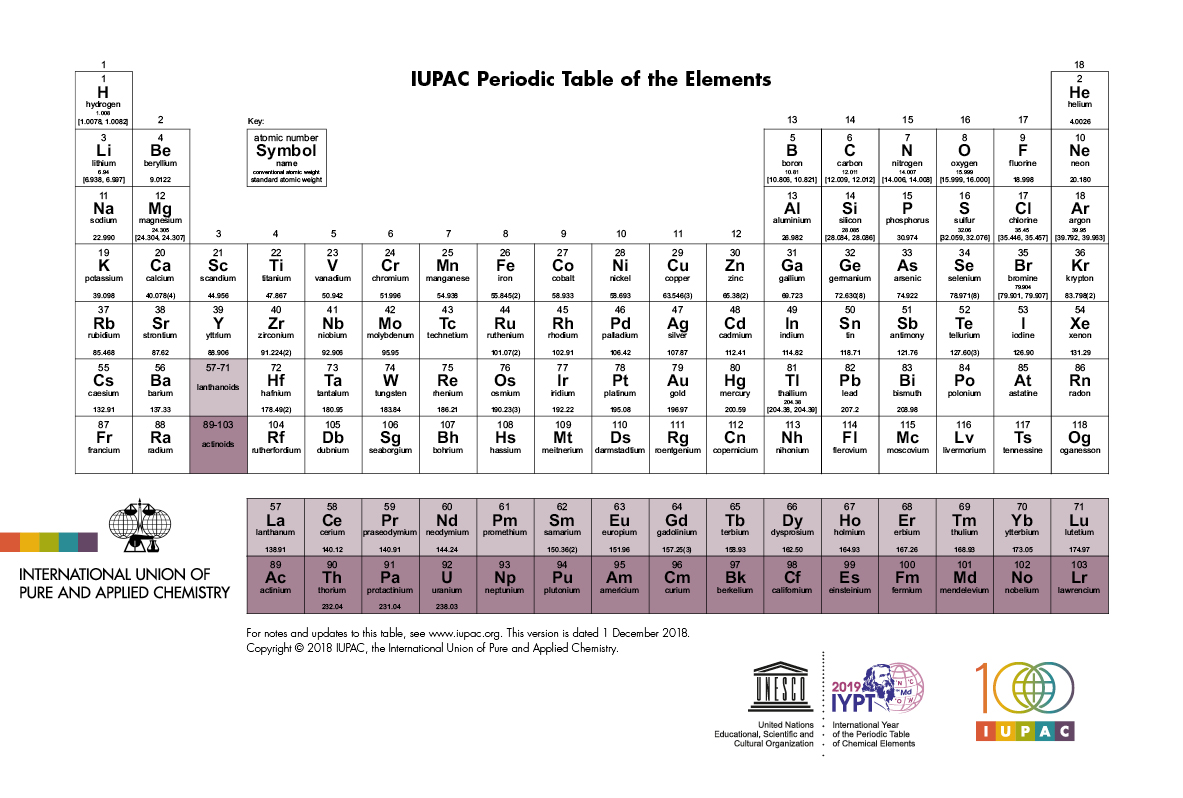
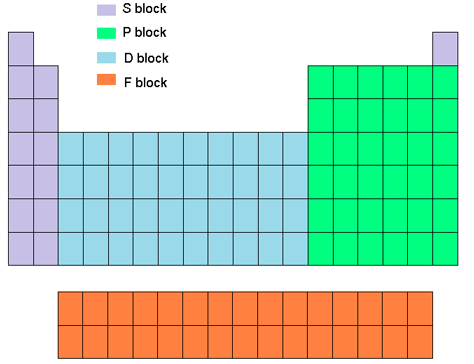
Group family periodic table definition. There are total 18 numbered groups in the modern periodic table however the f block columns between the group 2 and 3 are not numbered. Elements in this group have one orbital electron in the outer shell. Periodic table groups are columns of elements found in the modern periodic table.
A group is also known as a family of atoms in which elements are arranged within each group of the periodic table. Three systems have been used to number families and groups. There are 18 numbered groups in the periodic table.
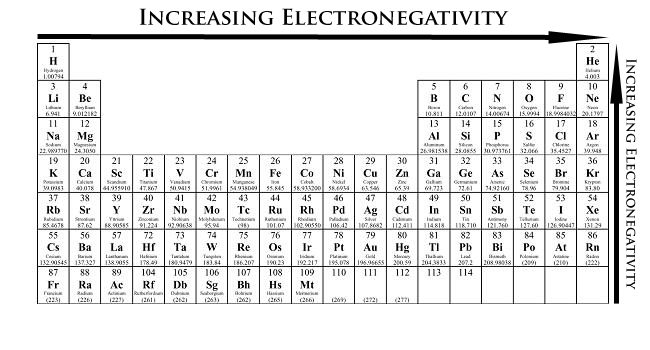
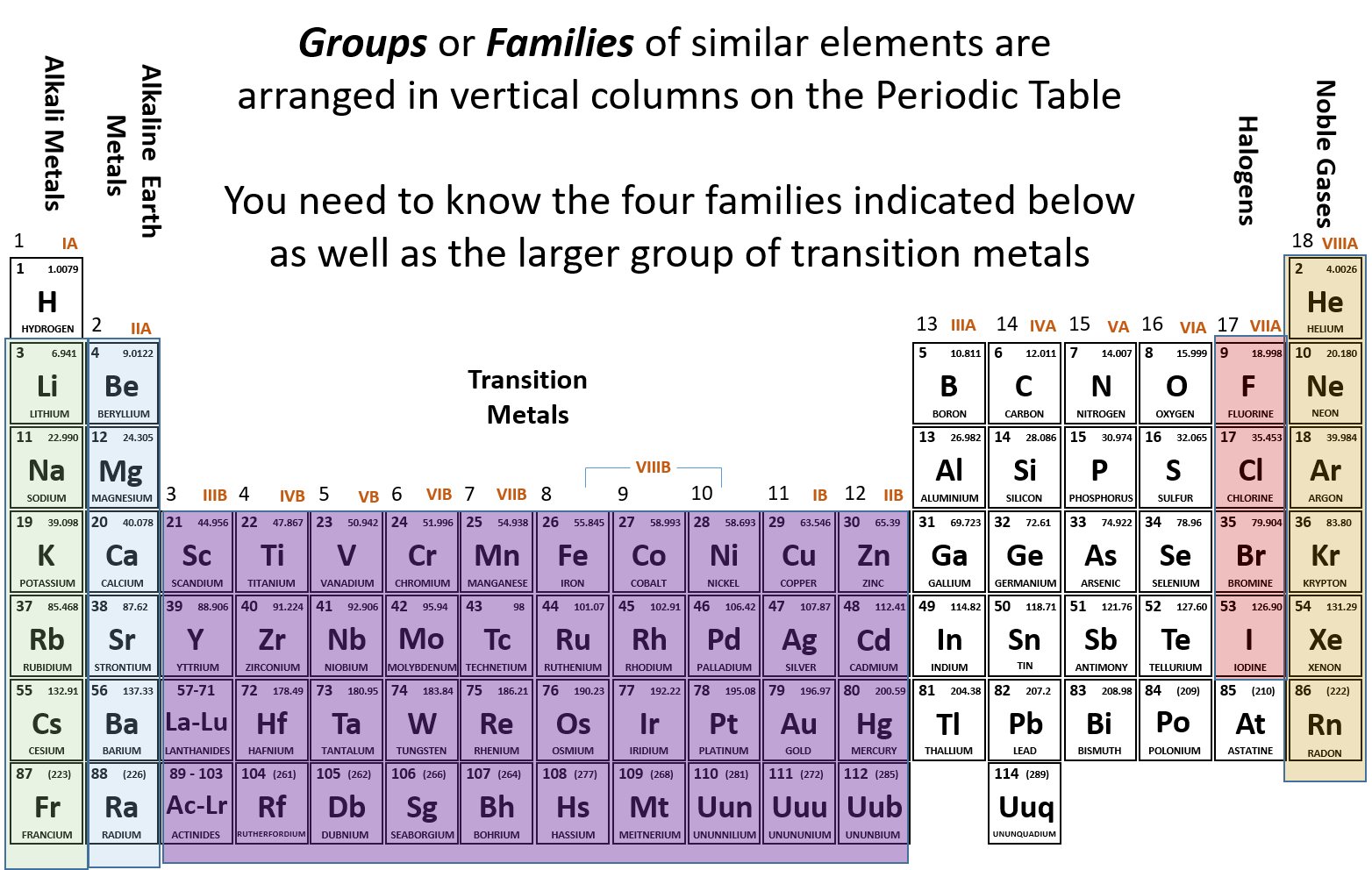
A group or family is the vertical columns on the periodic table of the elements. The vertical columns of elements are called groups or families. The older iupac system used roman numerals together with letters to distinguish between the left a and right b side of the periodic table.
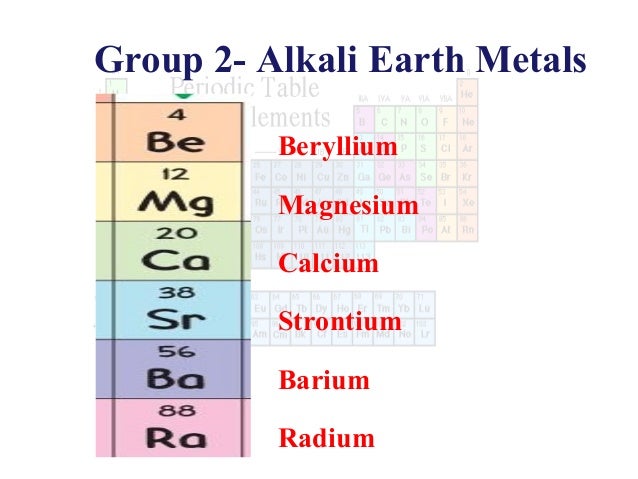
However some texts chemists and teachers distinguish between the two sets of elements. Group 1 is also known as the alkali metals or the lithium group. These elements have 8 electrons in the valence shell a complete octet.
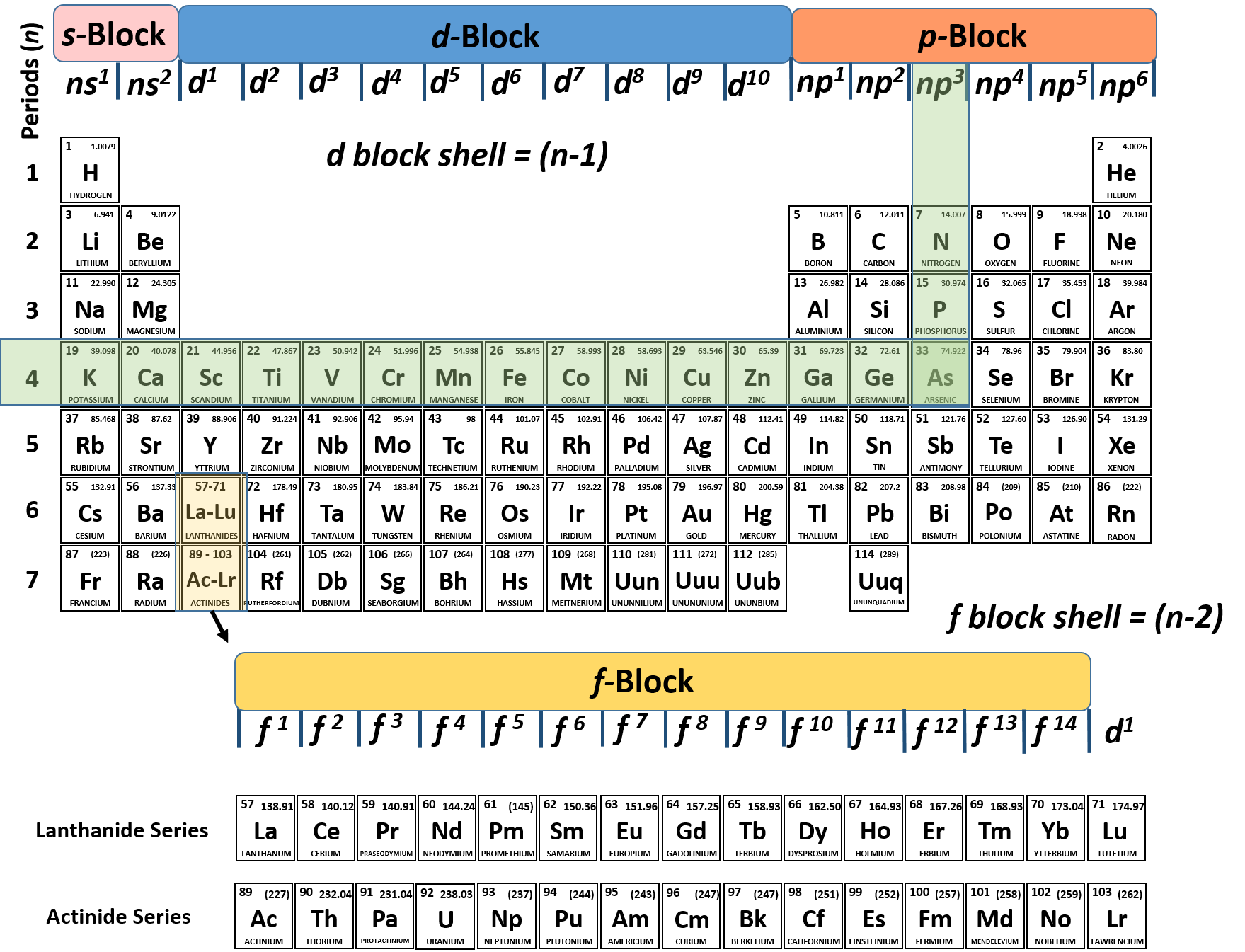
The f block columns between groups 3 and 4 are not numbered. Group 18 on the periodic table is also known as the noble gas family or noble gas group. The alkali and alkaline earth and transitions are all groups of the periodic table another point of view.
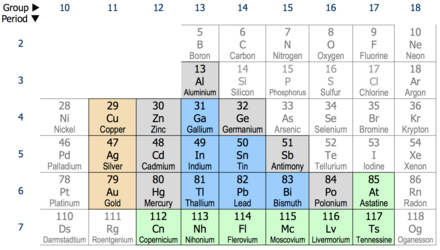
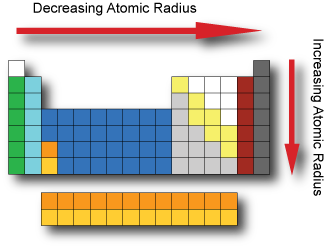
The same core charge as most chemical properties are dominated by the orbital location of the outermost electron. In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements. The most common way the periodic table is classified by metals nonmetals and metalloids.
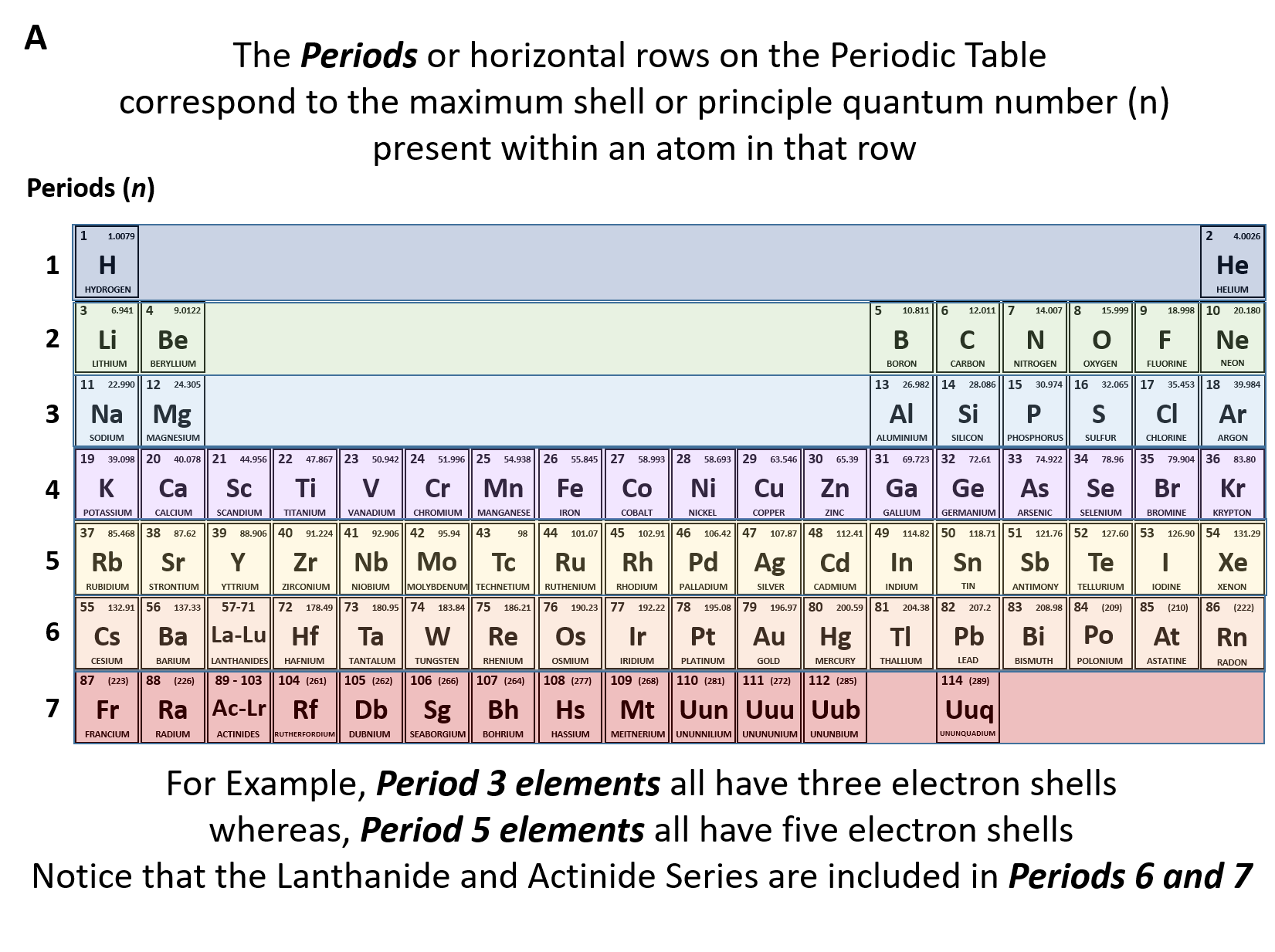
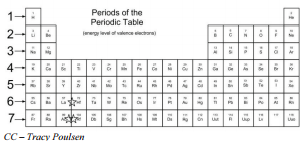
When the elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. In the periodic table of elements there are seven horizontal rows of elements called periods. Usually either family or group refers to one or more columns of the periodic table.
Group 16 is also known as the oxygen group or chalcogen family. Periods in the periodic table in each period horizontal row the atomic numbers increase from left. However some texts chemists and teachers distinguish between the two sets of elements.
Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number.
/periodic-table-165930186-590f2d703df78c92832fe141.jpg)



:max_bytes(150000):strip_icc()/PeriodicTableoftheElements-5c3648e546e0fb0001ba3a0a.jpg)


:max_bytes(150000):strip_icc()/CarbonGroup-56a12d313df78cf7726828af.png)
:max_bytes(150000):strip_icc()/the-periodic-table--digital-illustration--73016803-598b218ec41244001024af78.jpg)



:max_bytes(150000):strip_icc()/periodic-table-165930186-590f2d703df78c92832fe141.jpg)





:max_bytes(150000):strip_icc()/GettyImages-470784875-e7ea7f0da3d74a639d8e6a290afa000b.jpg)







:max_bytes(150000):strip_icc()/NitrogenGroup-56a12d313df78cf7726828b3.png)


0 Response to "Group Family Periodic Table Definition"
Post a Comment