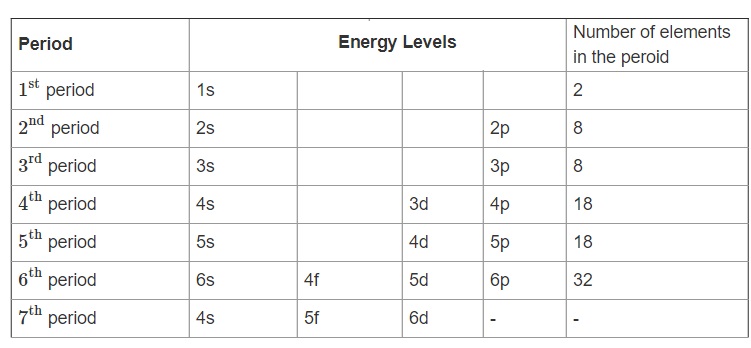
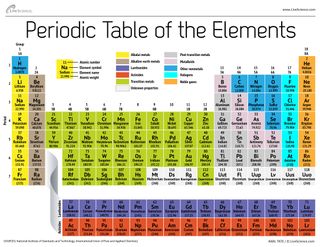
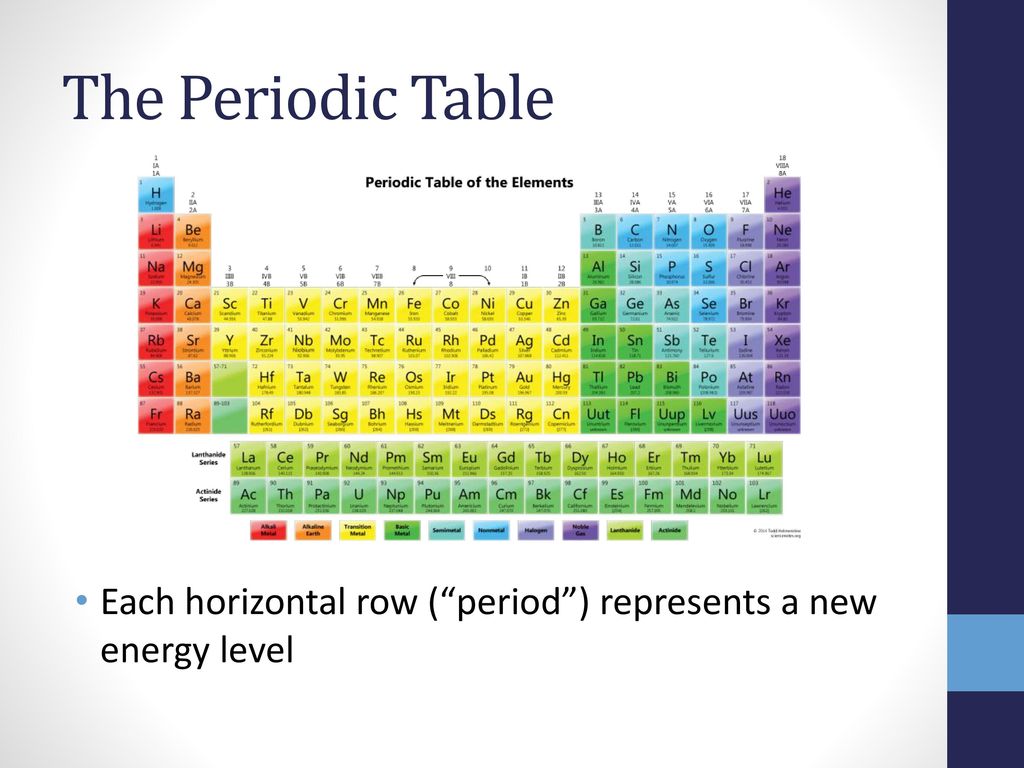
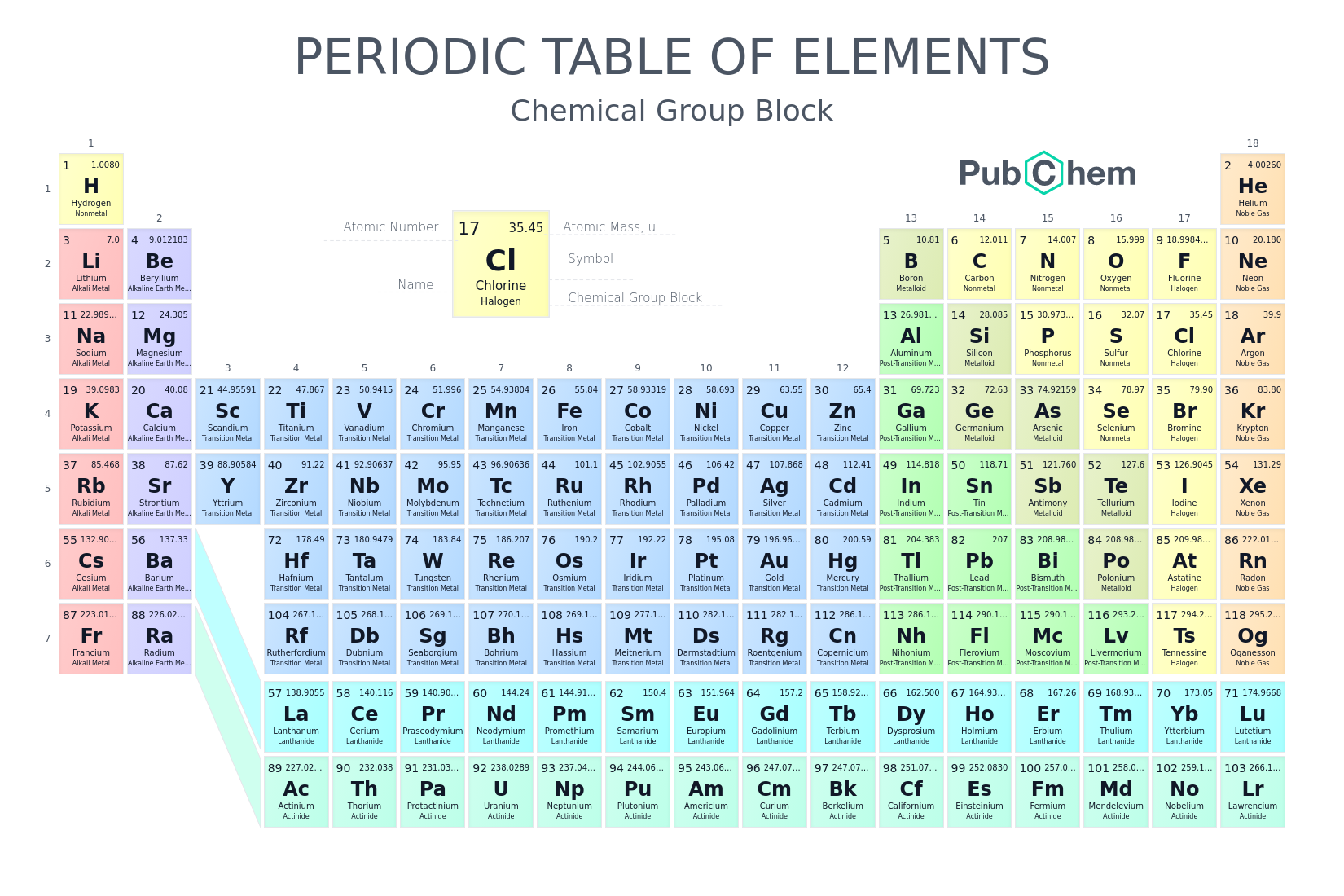
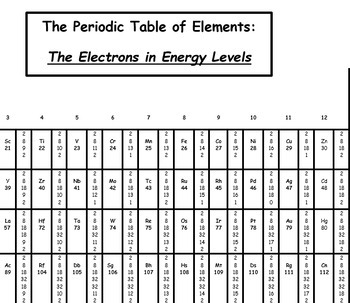
Periodic Table With Energy Levels Shown
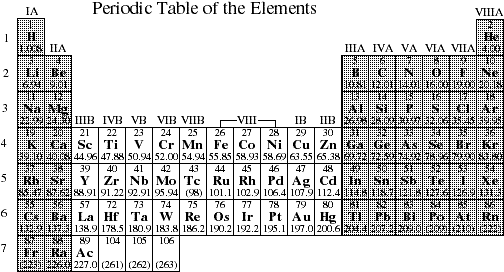
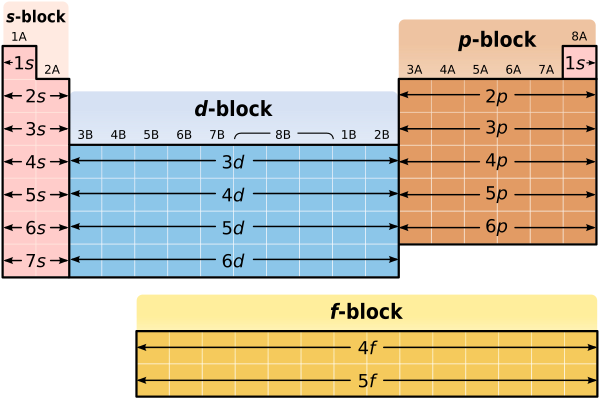
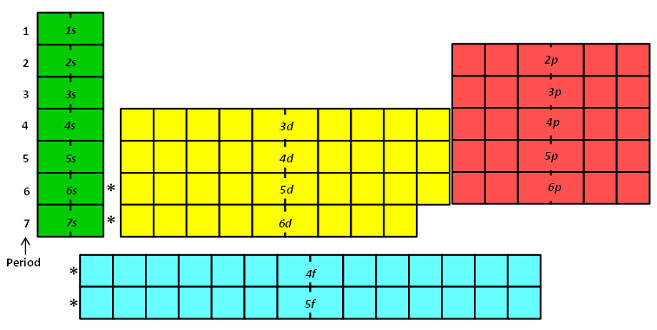
The periodic table is designed so that elements with the same valence electron configurations are in the same columns or groups. An elements period number represents the highest energy level that an electron in that element possesses.
Each energy level holds a certain number of electrons before electrons go into the next level.

Periodic table with energy levels shown. This table contains energy level models for the first 20 elements. Notice that all group 2 elements have 2 valence electrons giving a full s orbital for example. Because heliums energy level is filled with two electrons the atom is stable and does not react.
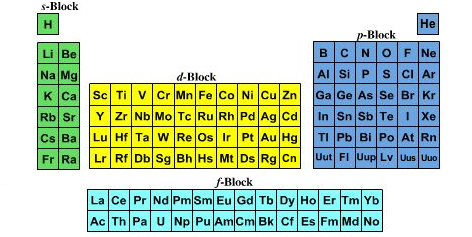
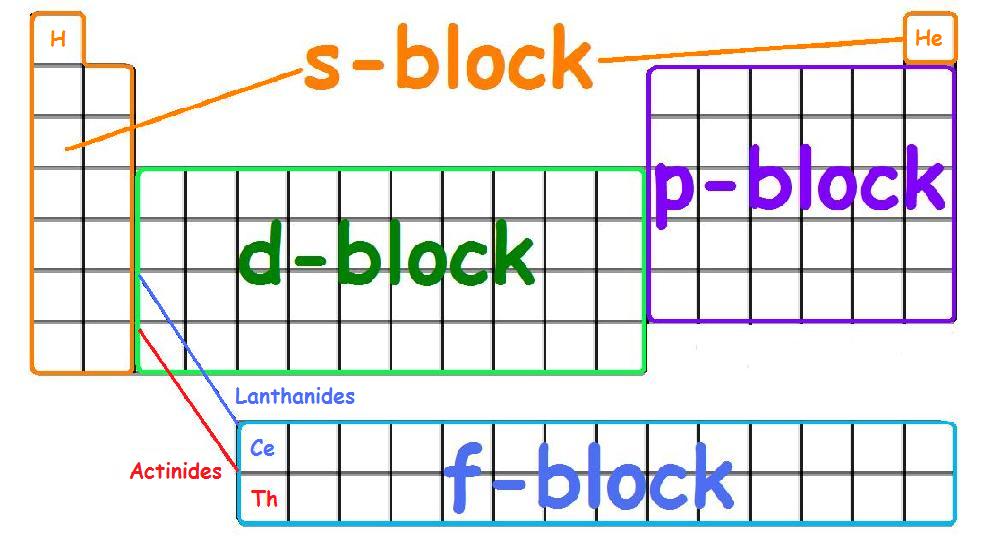
Explore project the periodic table of energy levels and discuss the arrangement of electrons as students complete their. The first two columns of the periodic table are known as the s block. There are only 18 groups in the periodic table that constitute the columns of the table.
The electrons are included only for the atoms at the beginning and end of each period. Lanthanoids and actinoids are numbered as 101 and 102 to separate them in sorting by group. This table shows the pattern in the periodic table that mendeleev developed and how the missing elements at that time could be predicted.
Helium has two electrons in the s orbital filling the energy level. This means that the valence electrons for these two columns exist in an s orbital. Periodic table of elements and x ray energies innovation with integrity handheld xrf 1 101 h 00007 hydrogen 2 400 he00002 helium 3 694 li 053 lithium 4 901 be 185 beryllium ka 0108.
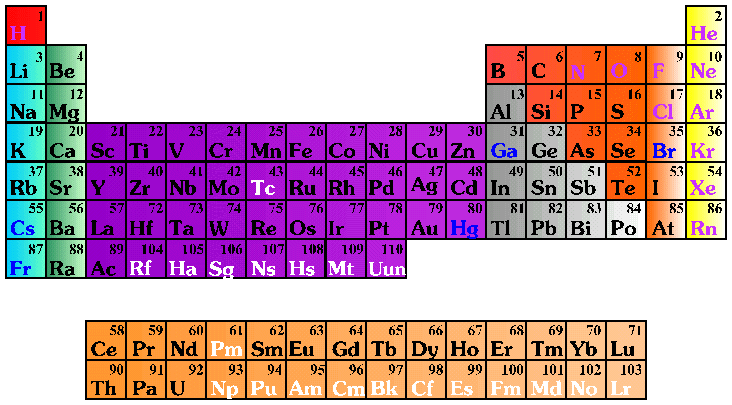
Elements found within the same column have electron distributions that are. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesthe structure of the table shows periodic trendsthe seven rows of the table called periods generally have metals on the left and nonmetals on the right. After the first level has two electrons the next electron goes into the second level.
Hydrogen and heliumelectrons go into the first energy level. Give each student a periodic table of energy levels activity sheet. Navigate by clicking the element on the table above or using the table below.
The first energy level only contains an s orbital. The periodic table of the elements with ionization energies 1 18 hydrogen 1 h 101 1312 2 alkali metals alkaline earth metals transition metals lanthanides actinides other metals metalloids semi metal first ionization nonmetals 694 halogens noble gases element name 80 symbol boron energy kjmol mercury hg 20059 1007 atomic lithium. So the periodic table is the best resource for the order in which orbitals are filled.
For example hydrogen has one electron in the s orbital. Interpreting the periodic table. Rows on the periodic table are referred to as periods while the columns on the periodic table are referred to as groups.
Electrons orbit the atoms nucleus in energy levels. Lanthanoids and actinoids are numbered as 101 and 102 to separate them in sorting by group. After all it was designed with.



















0 Response to "Periodic Table With Energy Levels Shown"
Post a Comment