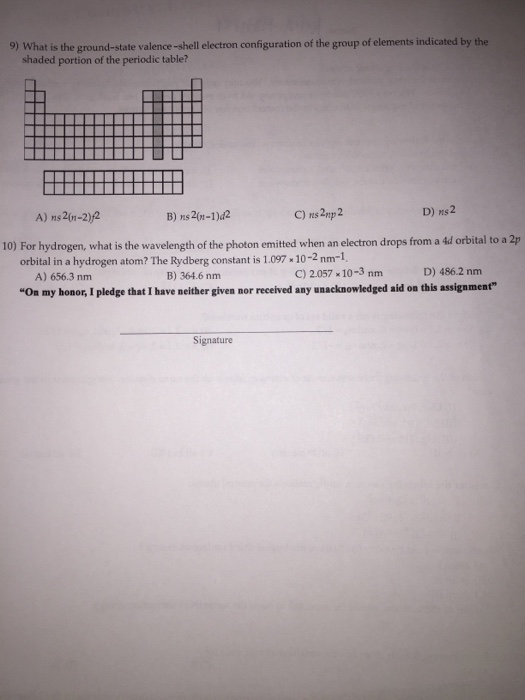
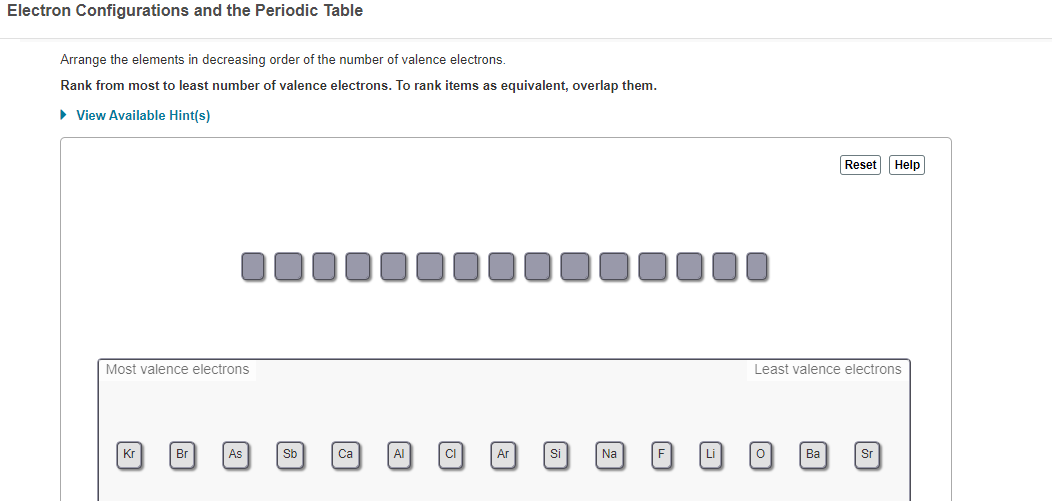
Periodic Table Valence Shell Electron Configuration
The other members of group 8 have a characteristic valence shell electron octet ns 2 np x 2 np y 2 np z 2. This article provides you with an electronic configuration chart for all these elements.
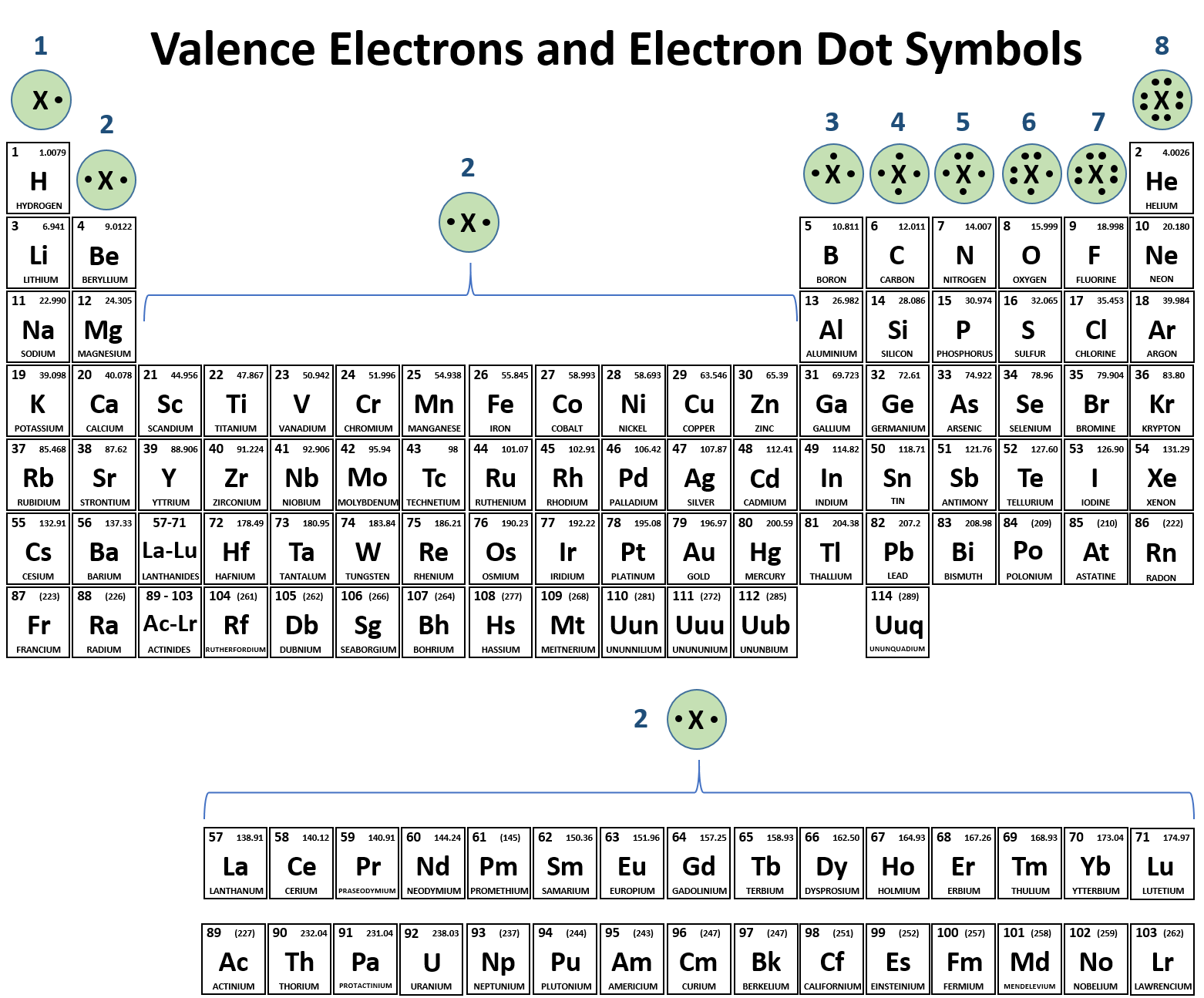
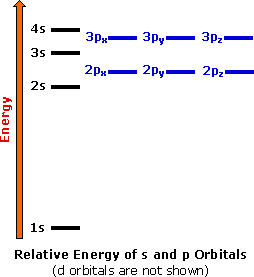
By definition valence electrons travel in the subshell farthest away from the nucleus of the atom.

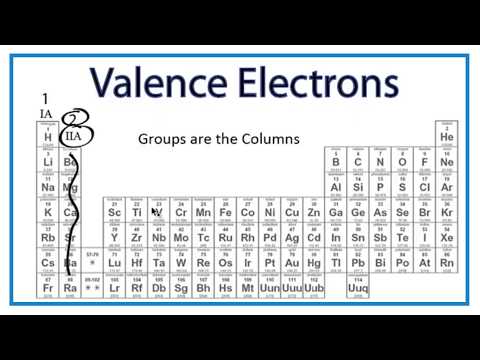
Periodic table valence shell electron configuration. First electrons filled in low energy levels and then move to higher energy level. How to figure valence of electrons in the periodic table. Group i the elements in that group have one valence electron in the outermost shell.
If youre seeing this message it means were having trouble loading external resources on our website. 4s 2 4p 6 xenon xe. Each electron shell is composed of one or more subshells.
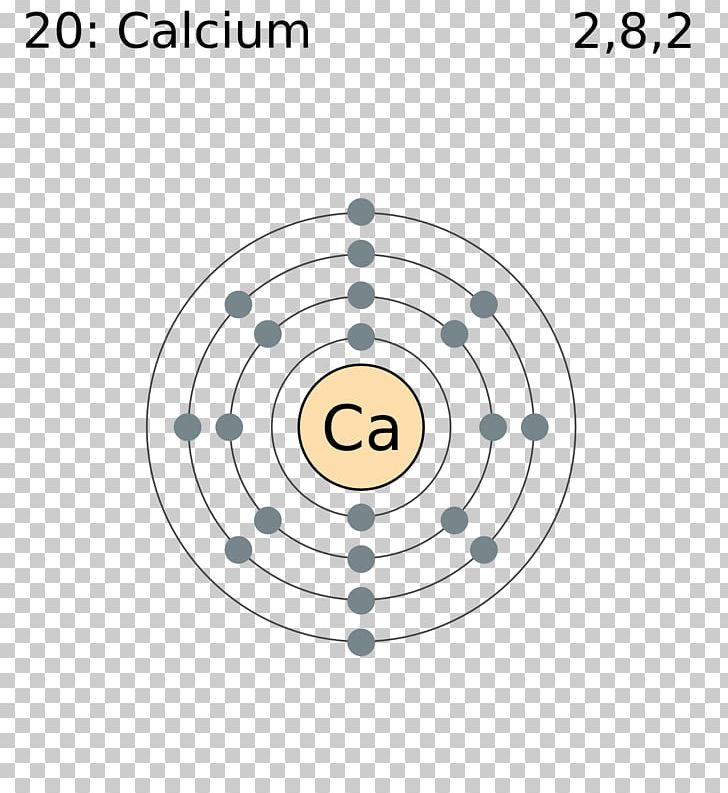
Please note that the number of outer shell electrons is the major determinant of the elements valence. Such an atom has the following electron configuration. Selenium on the periodic table example pageindex1.
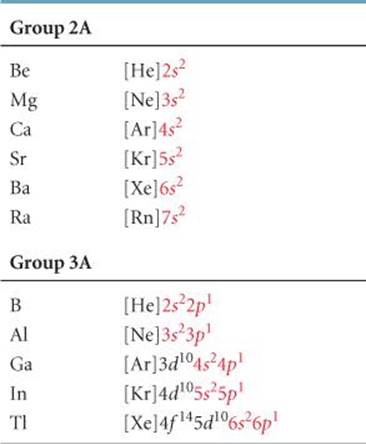
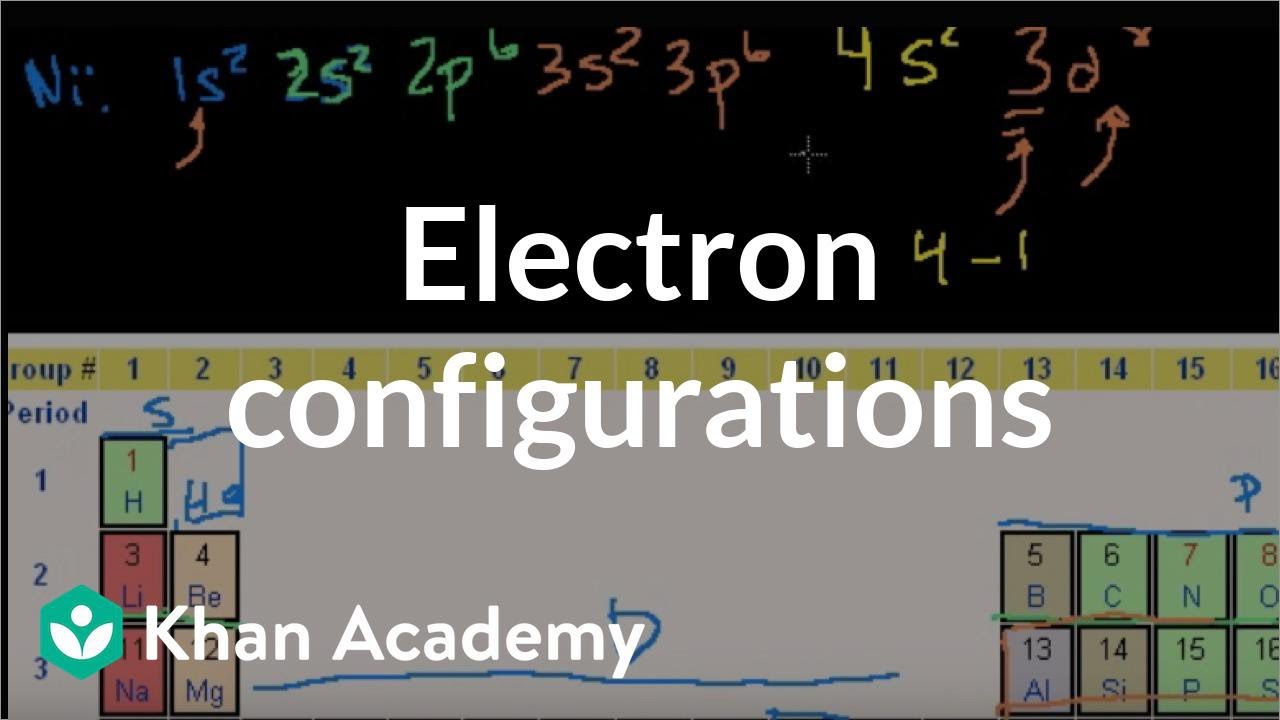
S 2 p 5. Using an elements position in the periodic table to predict its properties electron configuration and reactivity. Indeed the electron configuration of se is ar4s 2 3d 10 4p 4 as expected.
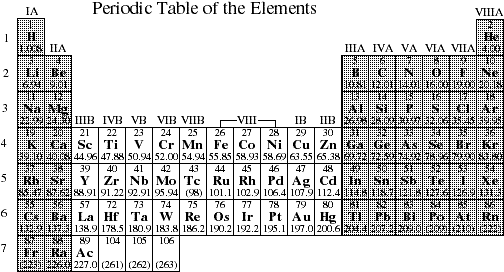
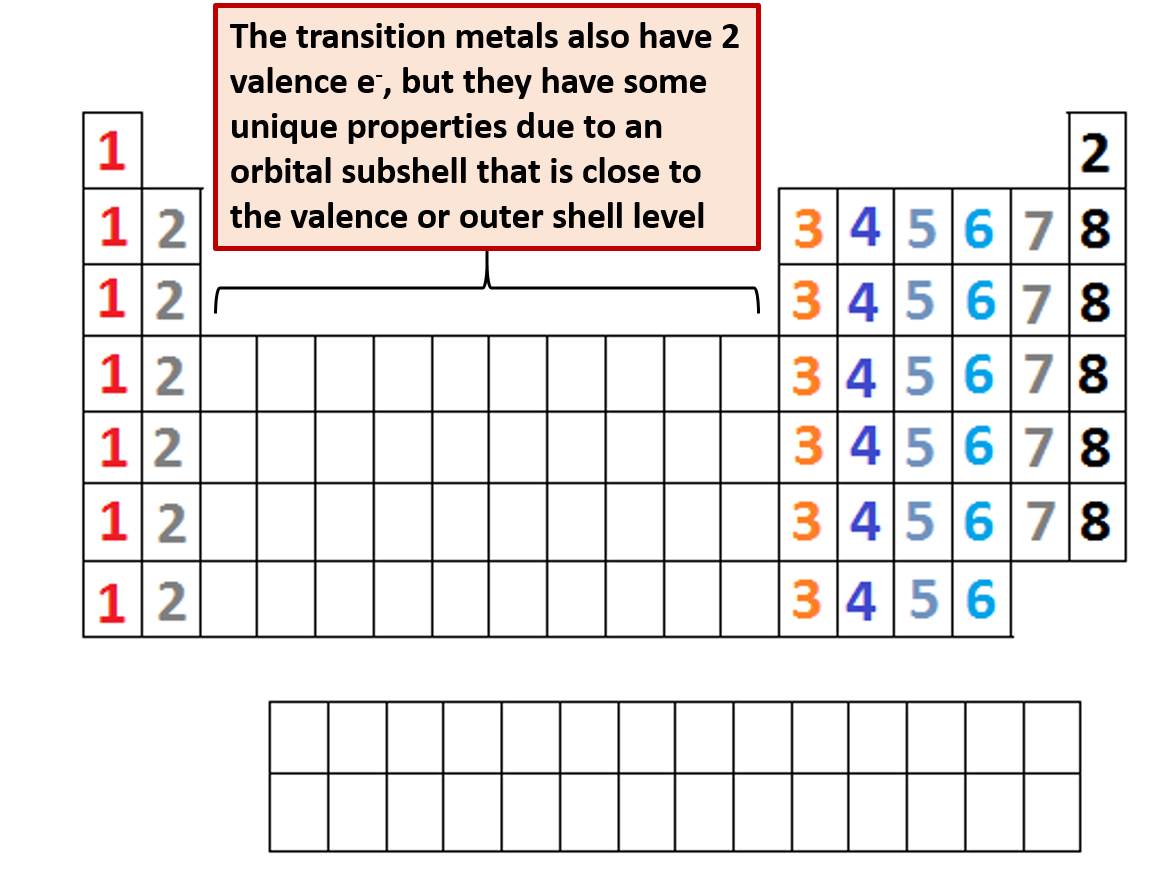
In the periodic table group indicates the number of valence electrons in the outermost shell. The periodicity of valence electrons this table illustrates a number of interesting and complicating features of electron configuration. There are 118 elements in the periodic table.
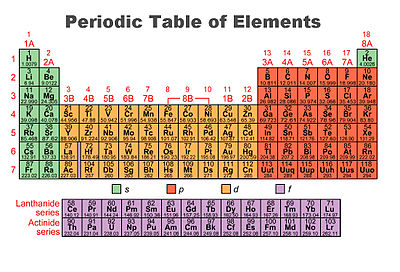
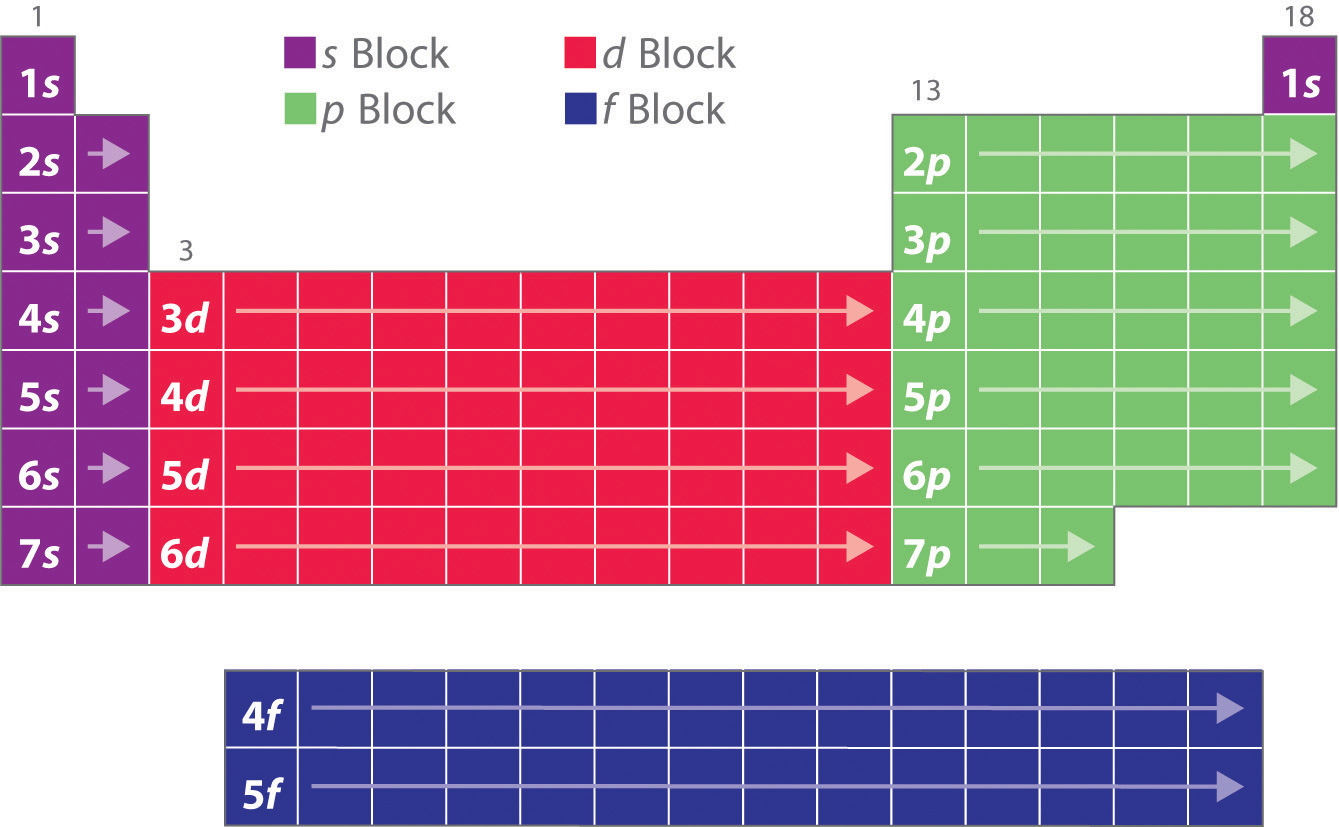
Electron shell configurations of the elements position in the periodic table based on electron shell configuration. Each element has a unique atomic structure that is influenced by its electronic configuration which is the distribution of electrons across different orbitals of an atom. The periodic table was designed with this feature in mind.
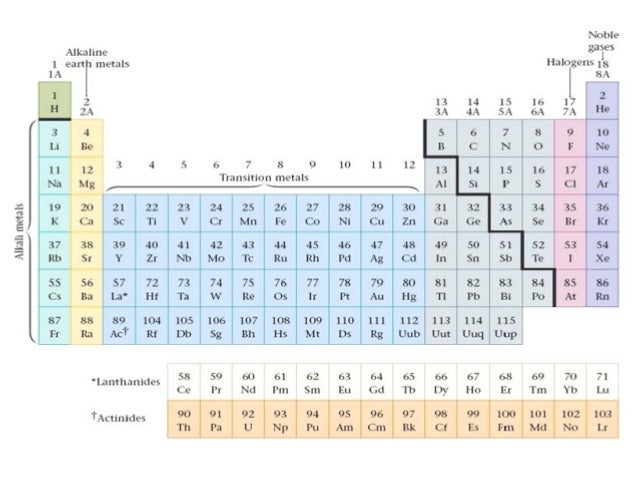
In the periodic table above these elements are colored beige. 6s 2 6p 6. From the elements position on the periodic table predict the valence shell electron configuration for each atom figure pageindex11.
This requires only one additional valence electron to form a closed shell. The bohr model and atomic orbitals. 5s 2 5p 6 and radon rn.
This image shows the entire periodic table with diagrammatic atoms and electron shells filling with movement through the. Electrons orbit around the nucleus of an atom at set energy levels known as principal energy levels or electron shells. This group of inert or noble gases also includes krypton kr.
If youre behind a web. Using an elements position in the periodic table to predict its properties electron configuration and reactivity. Valence electrons configuration and the periodic table in an atom electrons filled in different levels according to their energies.
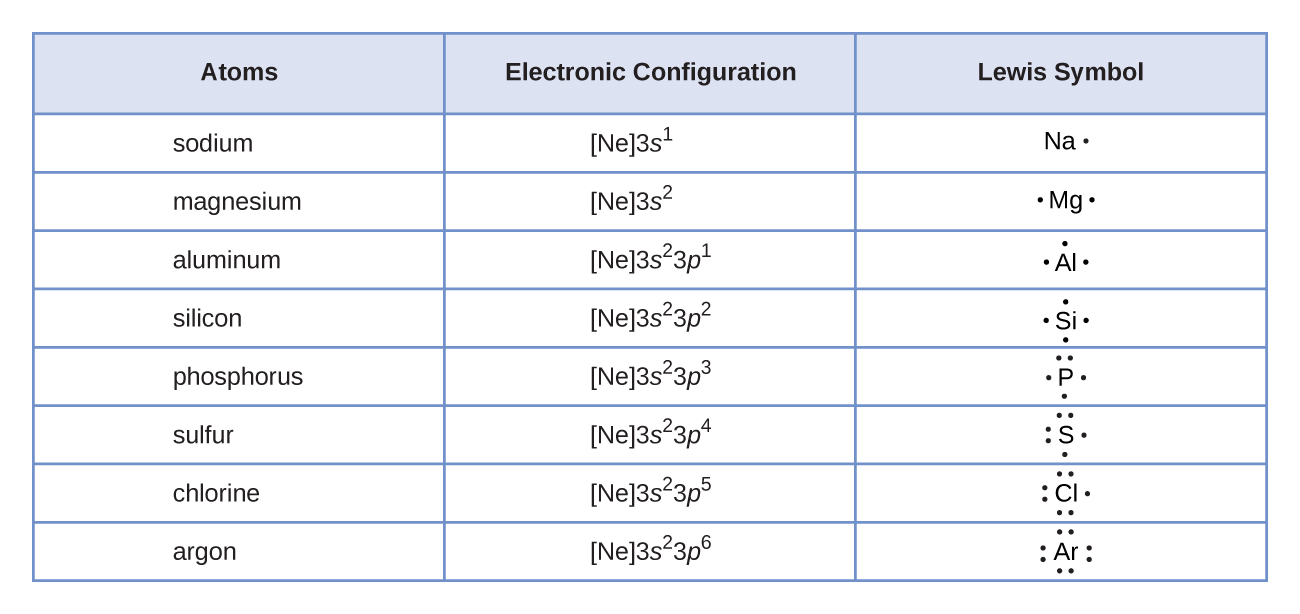
The outermost energy level in an atom is called as valence shell and electrons placed in this shell are known as valence electrons. Each element has a number of valence electrons equal to its group number on the periodic table. Atoms tend to accept or lose electrons if doing so will.
To form an ionic bond a halogen atom can remove an electron from another atom in order to form an anion eg f cl etc.






















0 Response to "Periodic Table Valence Shell Electron Configuration"
Post a Comment