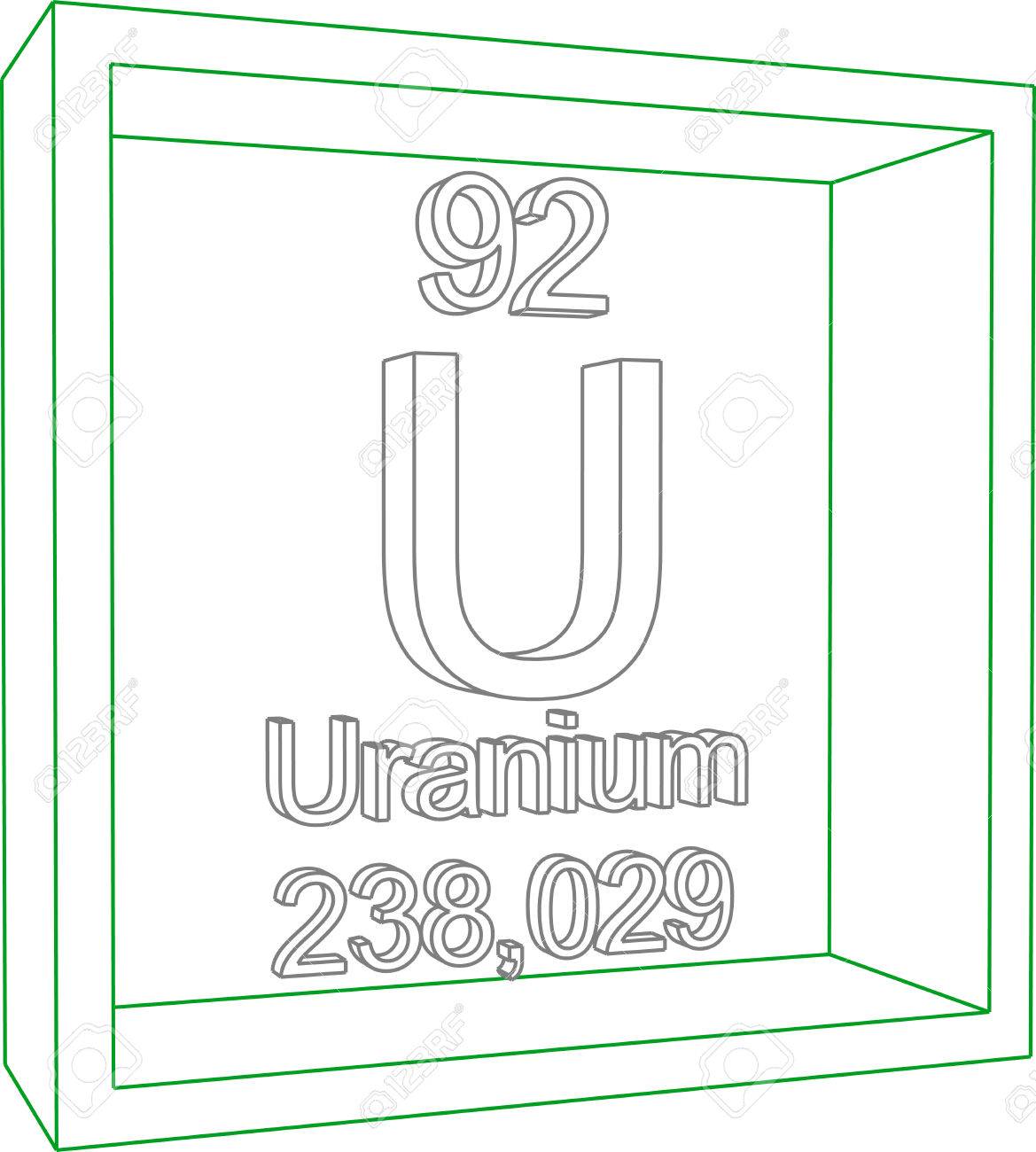
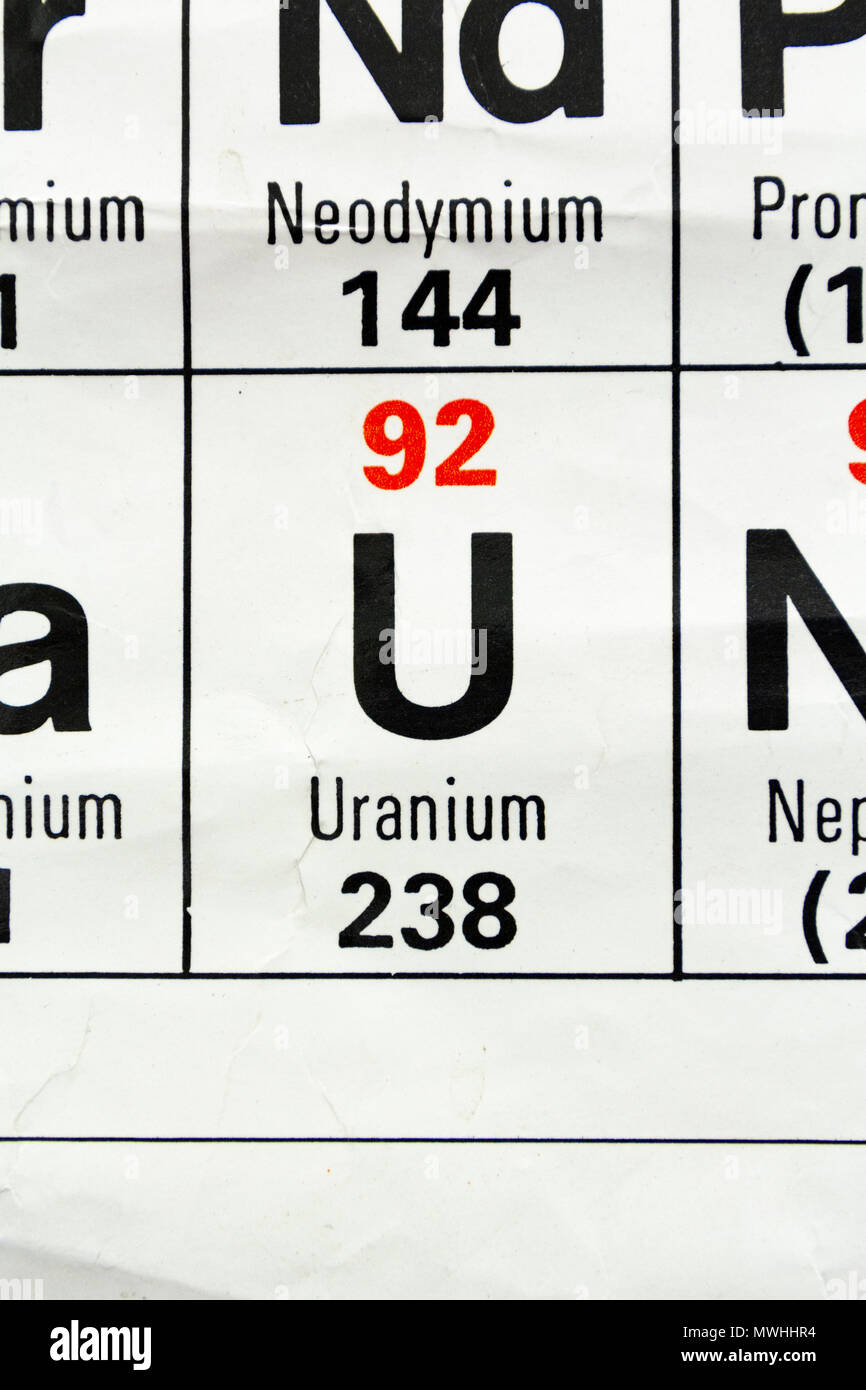
Uranium 238 Periodic Table
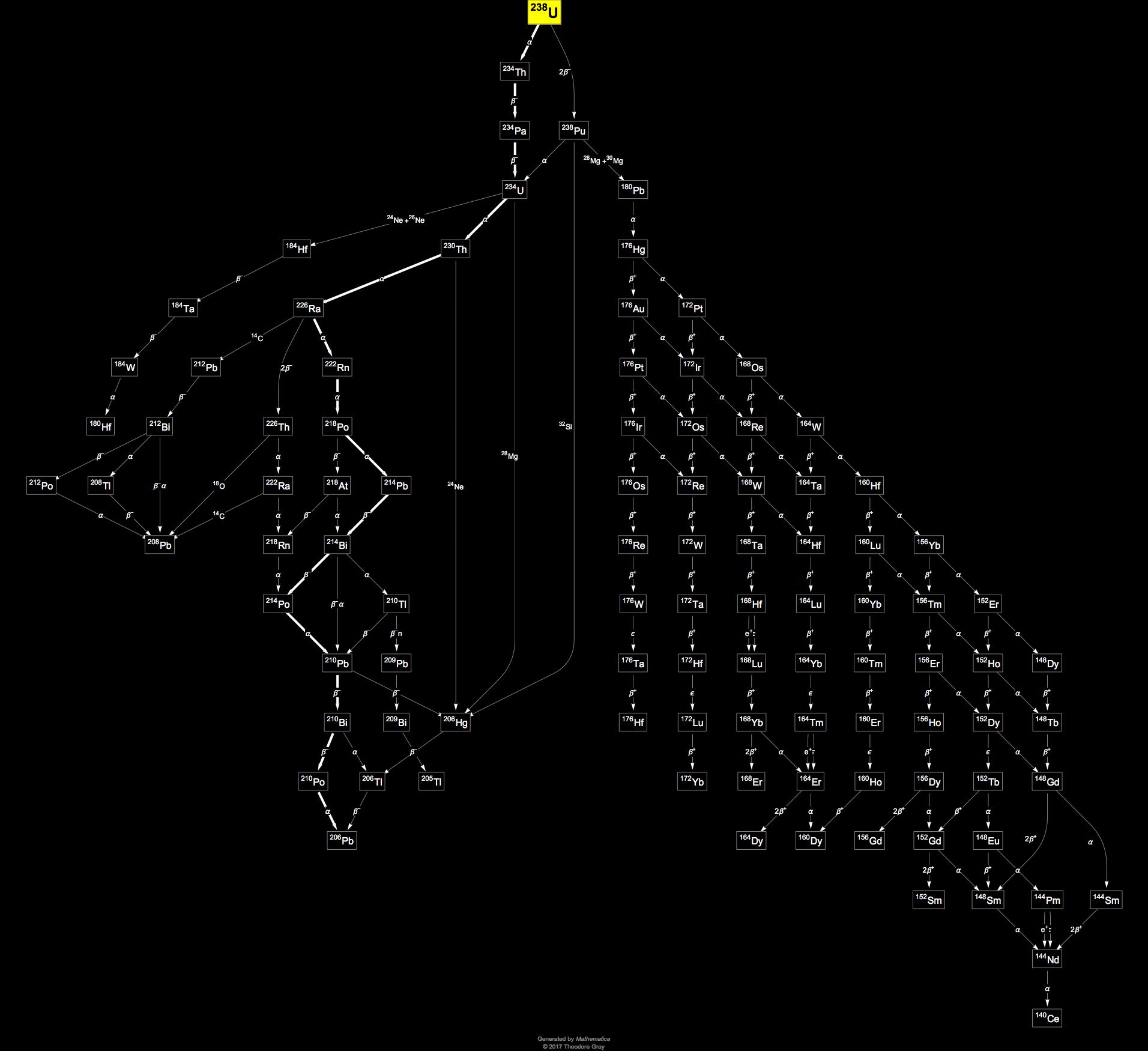
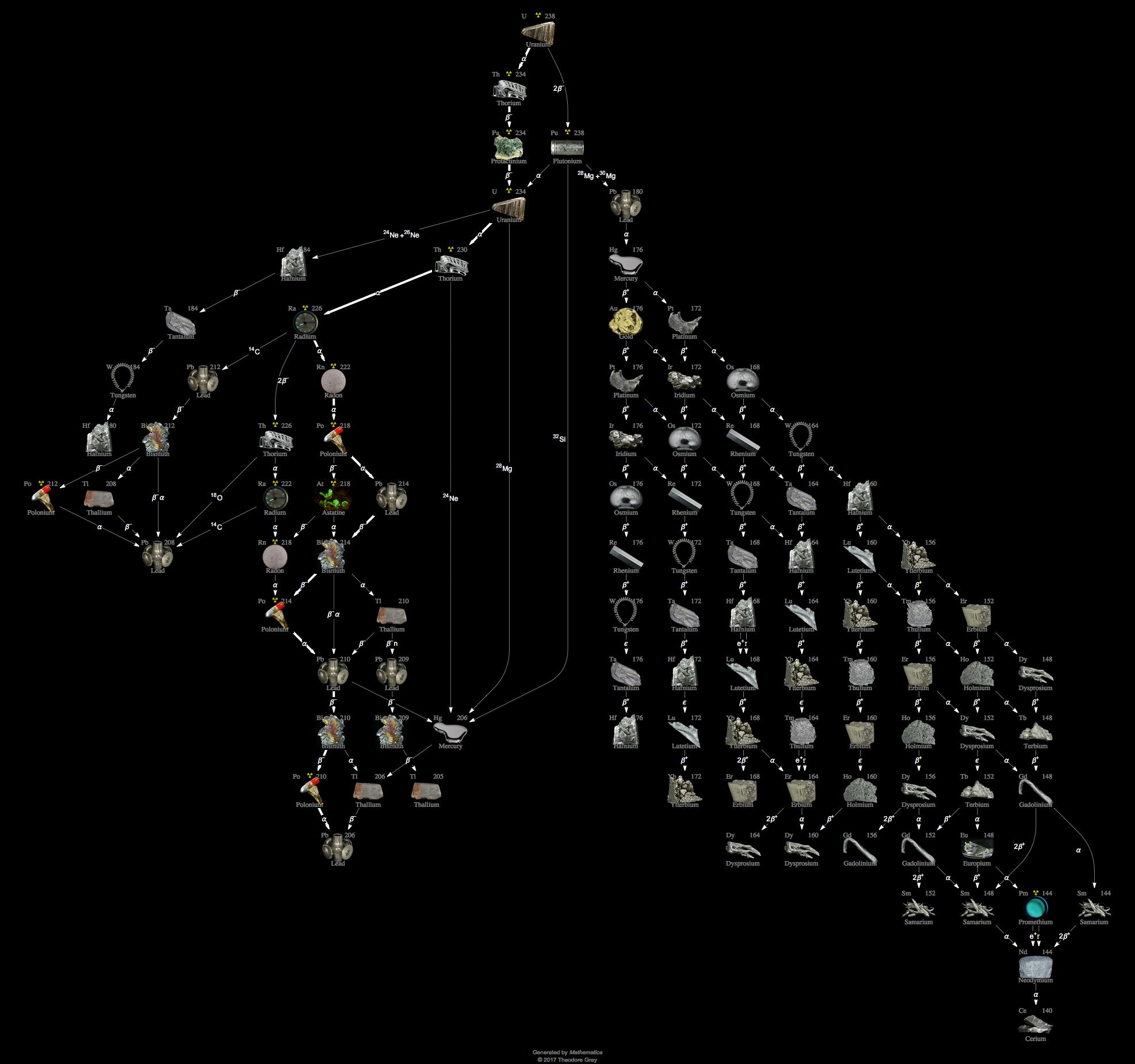
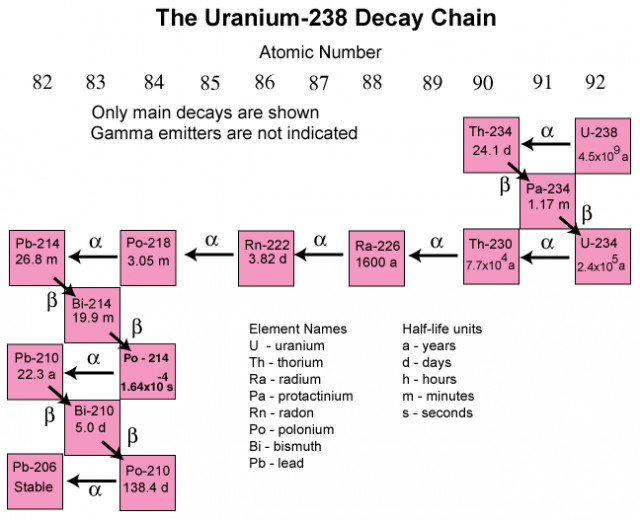
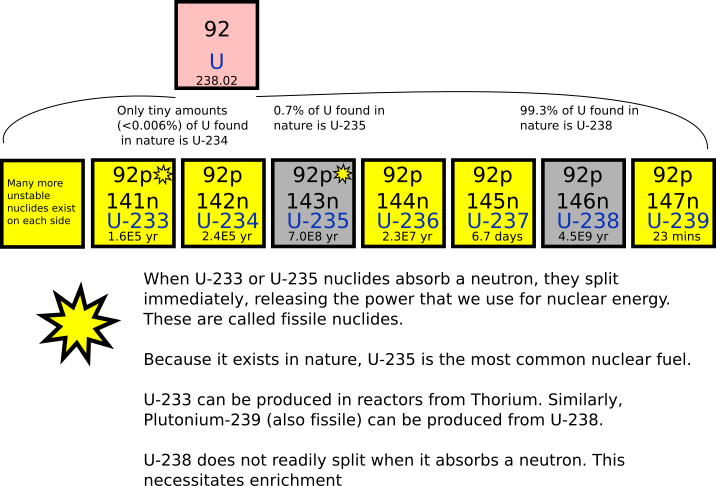
Uranium 238 is a fertile isotope. Isotopes of uranium click to see decay chain.
It is the most stable isotope with half life roughly equal to the age of earth.

Uranium 238 periodic table. The heat generated. All of the isotopes are radioactive. Uranium 235 is the only naturally occurring fissionable fuel a fuel that can sustain a chain reaction.
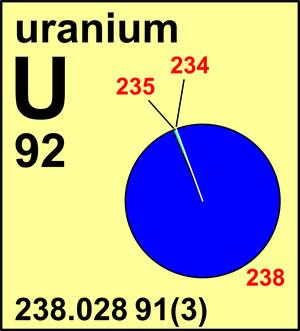
Naturally occurring uranium contains approximately 9928305 by weight u 238 07110 u 235 and 00054 u 234. Another isotope u 239 is created after the spontaneous fission of u 238. The half lives of its naturally occurring isotopes range between 159200 years and 45 billion years.
As a result of this equilibrium these two isotopes 238 u and 234 u contribute equally to the radioactivity of natural uranium. U 233 and uranium 237 are also formed from u 238 4. The percentage weight of u 235 in natural uranium depends on its source and may vary by as much as 01.
Uranium has sixteen isotopes. Other two major isotopes of uranium include uranium 235 which is 071 of naturally occurring uranium and uranium 234 00054 of naturally occurring uranium. Its density is about 70 higher than that of.
Periodic table uranium 238 which alone constitutes 9928 of natural uranium is the most common isotope of uranium in the nature. The chain reaction is carefully controlled using neutron absorbing materials. Uranium fuel used in nuclear reactors is enriched with uranium 235.
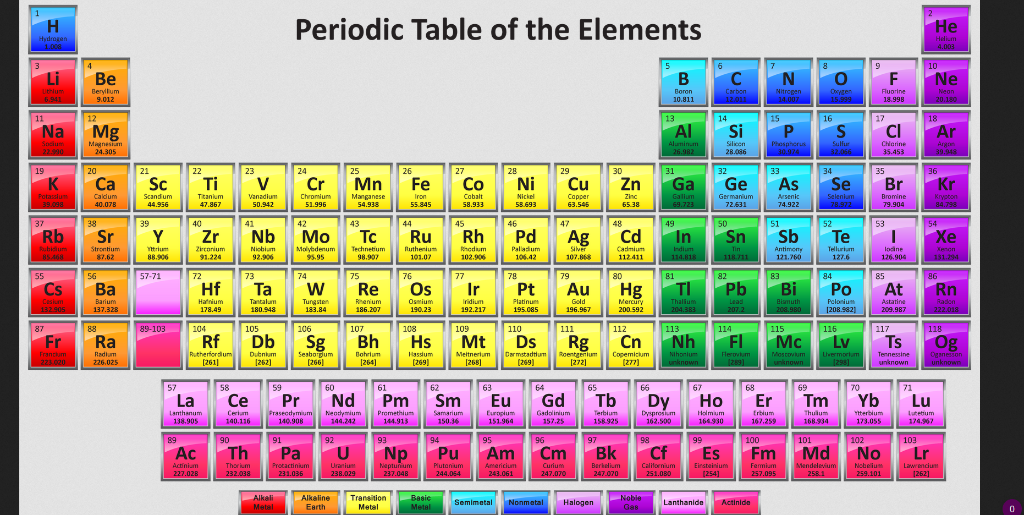
While its easy to find many elements on the periodic table uranium is below the main body of the table. Uranium is a chemical element with the symbol u and atomic number 92. Uranium 238 is the most commonly existing isotope of uranium.
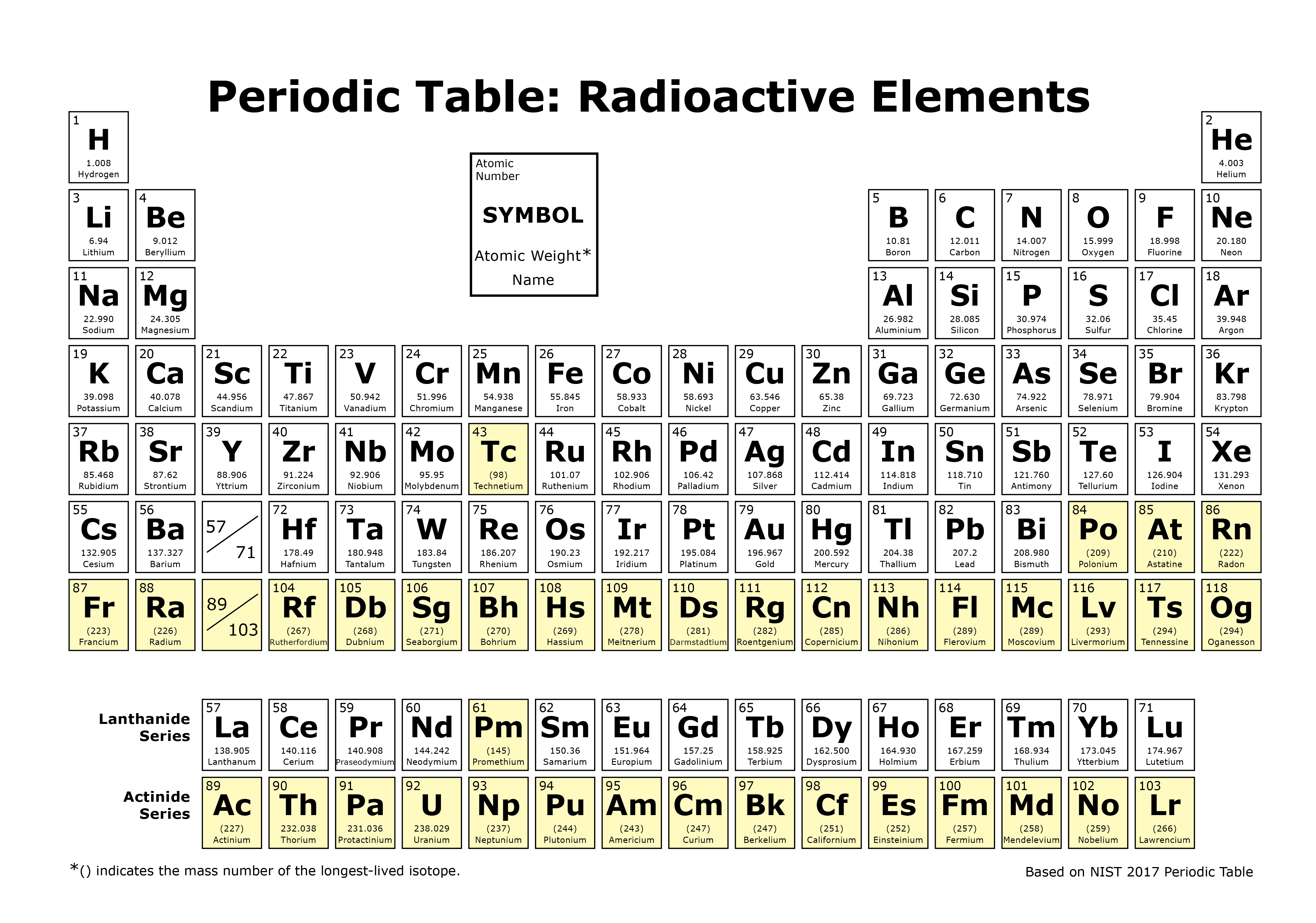
Uranium is a silvery white metal in the actinide series of the periodic table. These elements are still listed according to increasing atomic number but they are taken out of the table and placed below it because the lanthanides and actinide are transition metals. Uranium is weakly radioactive because all isotopes of uranium are unstable with half lives varying between 159200 years and 45 billion years.
Full descriptions from write up sources. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search. In a natural sample of uranium these nuclei are present in the unalterable proportions of the radioactive equilibrium of the 238 u filiation at a ratio of one atom of 234 u for about 18 500 nuclei of 238 u.
Naturally occurring uranium consists of 99 uranium 238 and 1 uranium 235. It is a silvery grey metal in the actinide series of the periodic tablea uranium atom has 92 protons and 92 electrons of which 6 are valence electronsuranium is weakly radioactive because all isotopes of uranium are unstable. Uranium has the highest atomic weight of the primordially occurring elements.
217 u 218 u 219 u 220 u 221 u 222 u 223 u 224 u 225 u 226 u 227 u 228 u 229 u 230 u 231 u 232 u 233 u 234 u 235 u 236 u 237 u 238 u 239 u 240 u 241 u 242 u.

























0 Response to "Uranium 238 Periodic Table"
Post a Comment