Periodic Table Rows Vs Columns
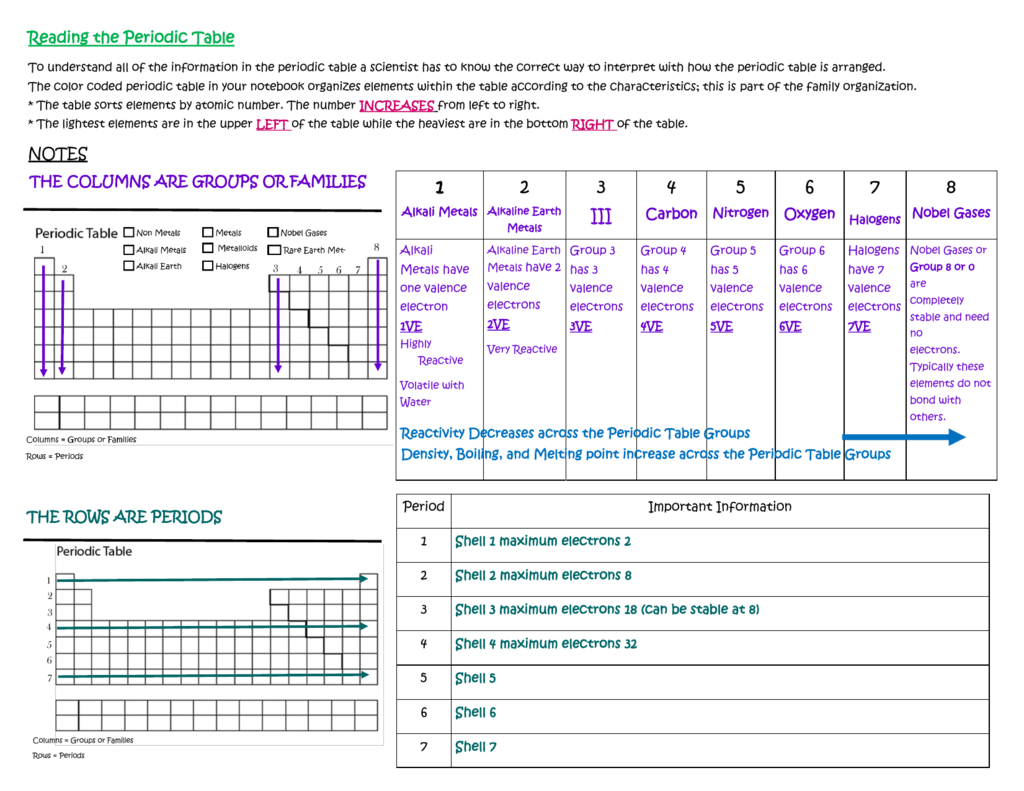
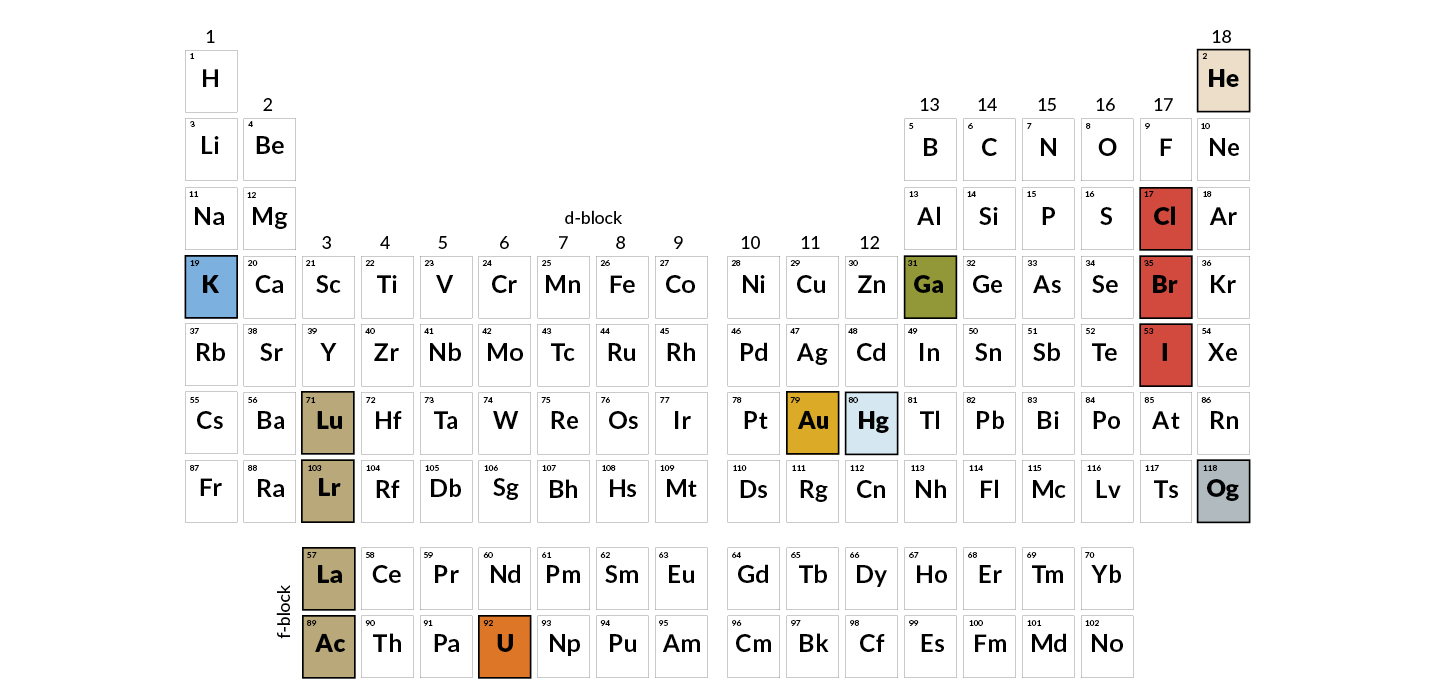
The number of valence electrons increases from left to right in the period. As with any grid the periodic table has rows left to right and columns up and down.
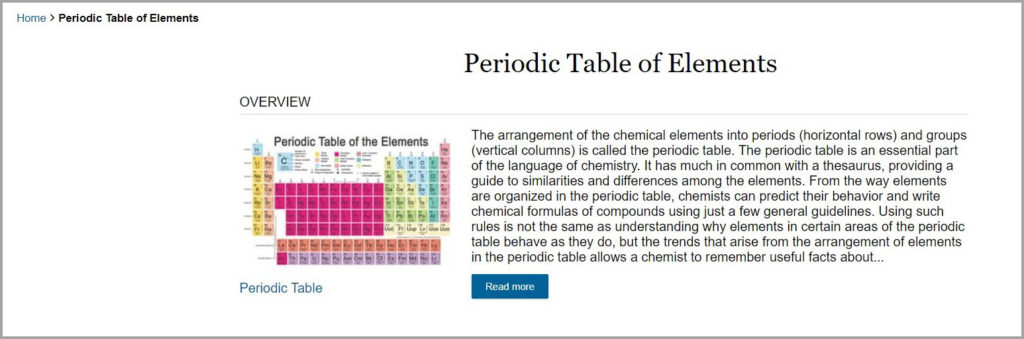
Periodic table showing hydrogen and helium as two top elements arrangement of elements in the rows periodic table the periodic table is divided into groups columns and periods rows image of periodic table showing groups as vertical columnds.

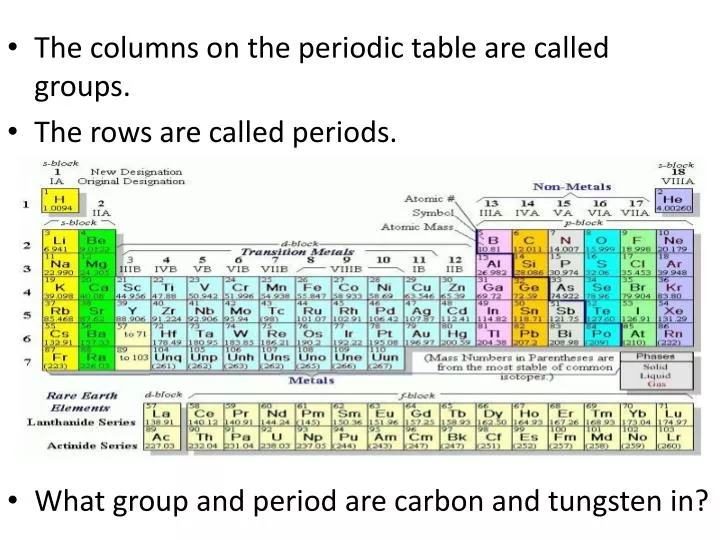
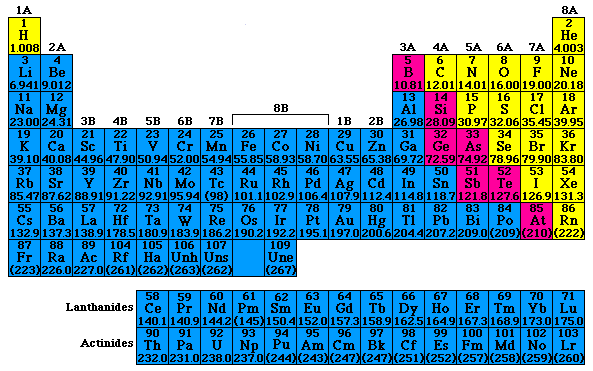
Periodic table rows vs columns. Elements as building blocks the periodic table is organized like a big grid. The rows on the periodic table are called periods. The most common way the periodic table is classified by metals nonmetals and metalloids.
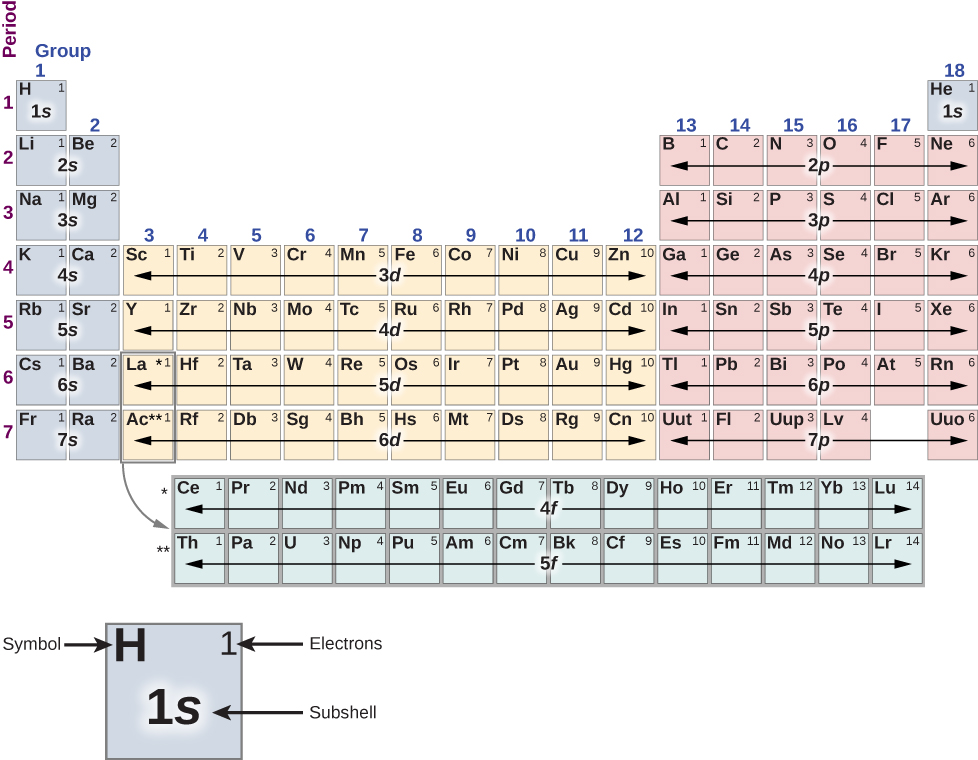
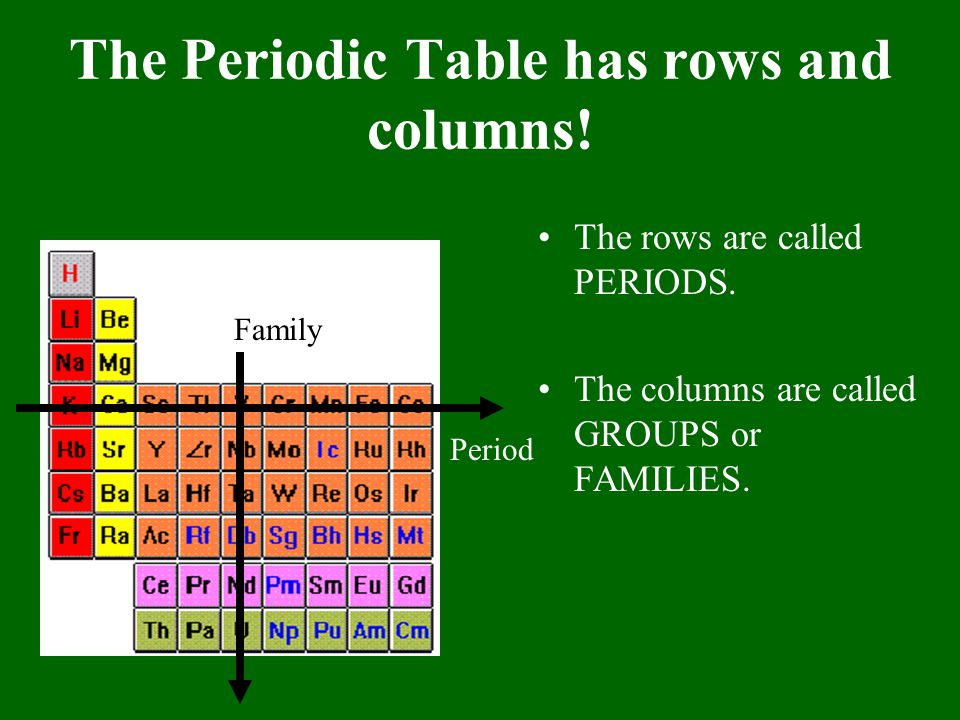
When you look at a periodic table each of the rows is considered to be a different period. Periods are horizontal rows across the periodic table while groups are vertical columns down the table. In the periodic table elements have something in common if they are in the same row.
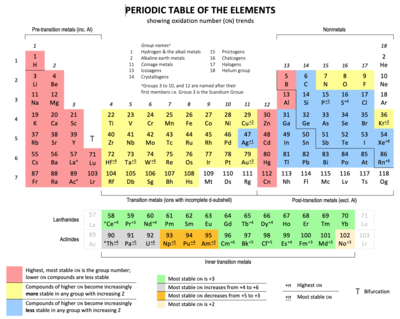
Periodic table comprises of rows and columns like in an organised manner. Conclusion even though they skip some squares in between all of the rows go left to right. Atomic number increases as you move down a group or across a period.
Each row and column has specific characteristics. The periodic table of elements is a large table in which each and every known chemical element is placed in a specific location considering the. However there exists a fine line of difference between rows and columns which has been explained in the given article in detail.
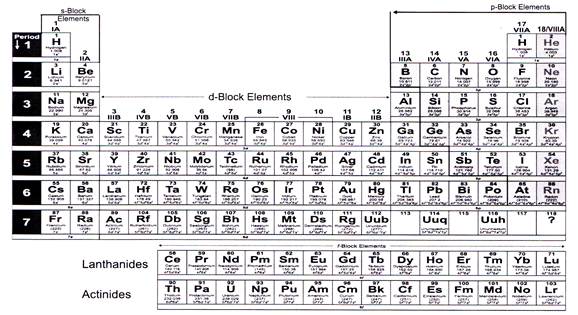
Each element is placed in a specific location because of its atomic structure. All the elements in a period have valence electrons in the same shell. Periods in the periodic table in each period horizontal row the atomic numbers increase from left.
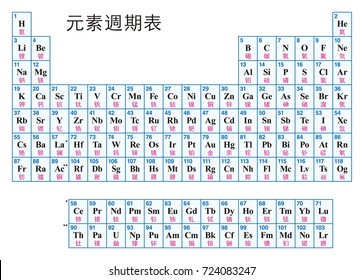
Hence it is called a table. The key difference between periods and groups is that the periods are horizontal rows whereas the groups are the vertical columns in the periodic table of chemical elementsthere are 7 major periods and 18 groups in the periodic table of elements. Periodic table columns vs rows.
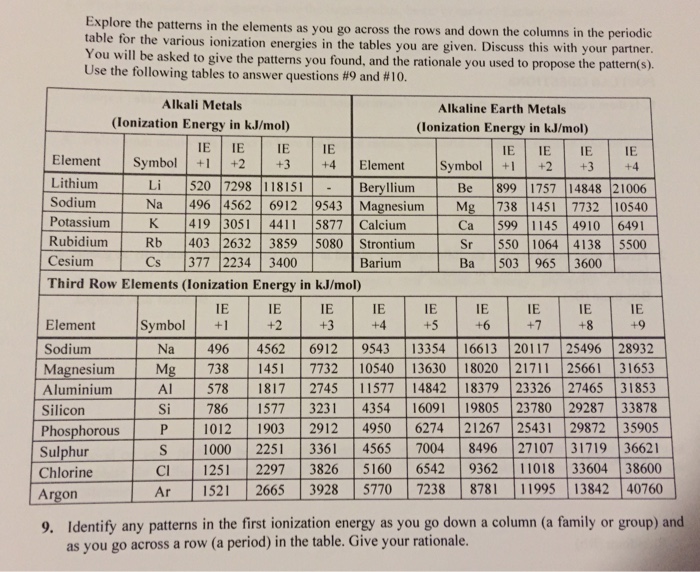
Groups and periods are two ways of categorizing elements in the periodic table. In the periodic table of elements there are seven horizontal rows of elements called periods. In zeilen und spalten von zellen zu arrangieren.
Html tabelle die html tabellen ermaglicht web autoren um daten wie texte bilder links andere tabellen etc. The vertical columns of elements are called groups or families. When the shell is full a new row is started and the process repeats.
People often misconstrue rows for columns as they are used in the matrix spreadsheets and classroom settings too for the purpose of bifurcating categories groups types and so on. Asked in chemistry periodic table elements and compounds what are the columns and rows of.












:max_bytes(150000):strip_icc()/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)










/186810031-56a130cd5f9b58b7d0bce8ee.jpg)

0 Response to "Periodic Table Rows Vs Columns"
Post a Comment