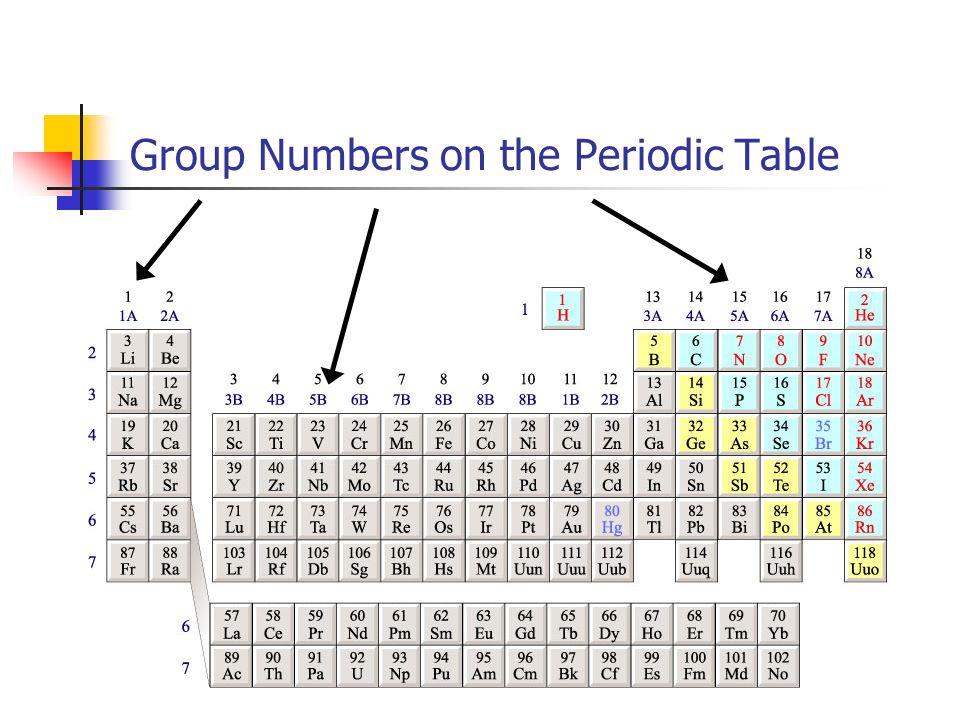
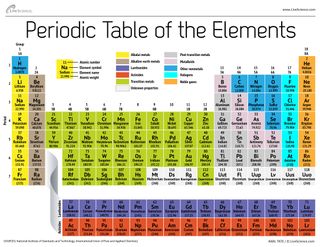
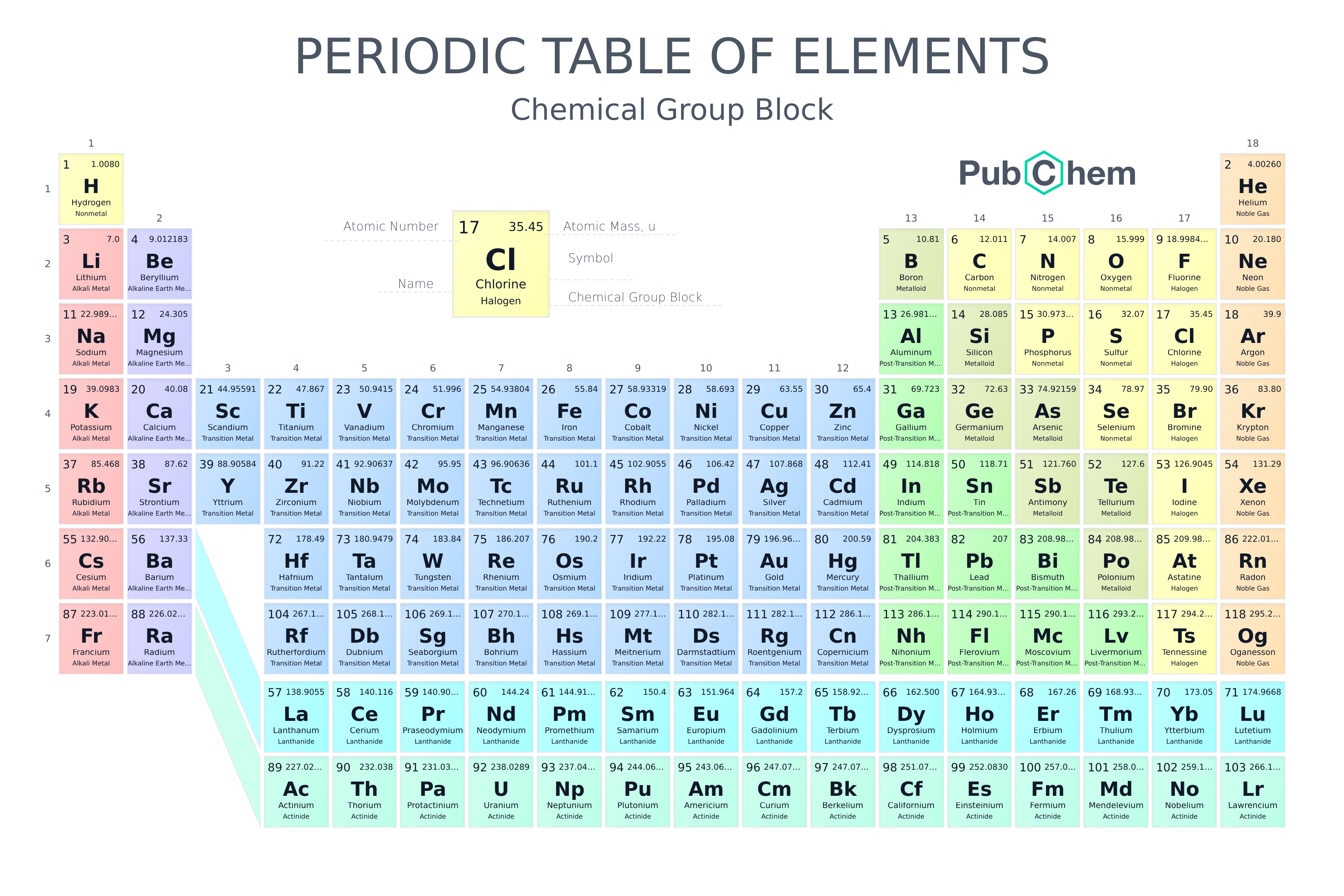
Periodic Table Group Numbers
Groups 1 2 termed s block elements. There is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties.
At this level all we do in addition to the elements in this bit of the periodic table is to look in more detail at the whole of the groups 1 2 14 17 and 18.

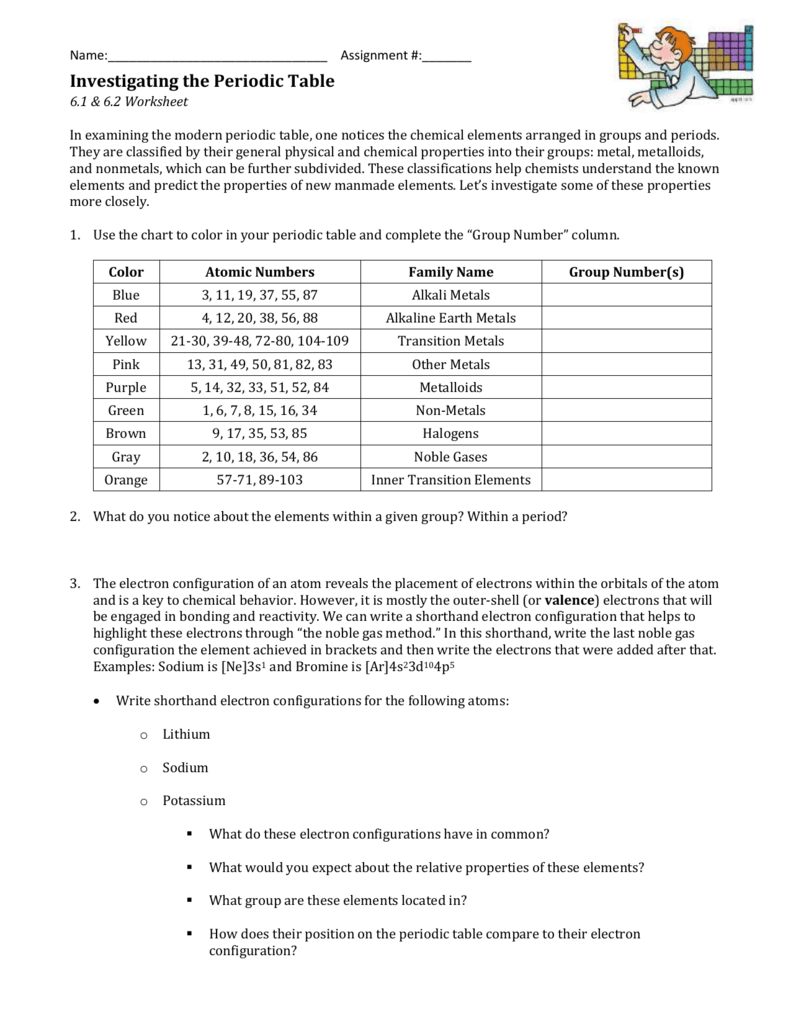
Periodic table group numbers. The elements in a group share the same number of valence electrons and thus have many common chemical and physical properties. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesthe structure of the table shows periodic trendsthe seven rows of the table called periods generally have metals on the left and nonmetals on the right. Groups 3 11 are termed transition elements.
The atomic number of an element is the same as the number of protons in that particular nucleus of an atom. For these elements the weight value shown represents the mass number. The f block columns between groups 3 and 4 are not numbered.
There are 18 numbered groups in the periodic table. The periodic table is a tabular arrangement of the chemical elements. Many periodic tables list numbers for element groups which are columns of the periodic table.
However there wasnt always a standard method of numbering groups so this can be confusing when consulting older tables. Lanthanoids and actinoids are numbered as 101 and 102 to separate them in sorting by group. Full descriptions from write up sources.
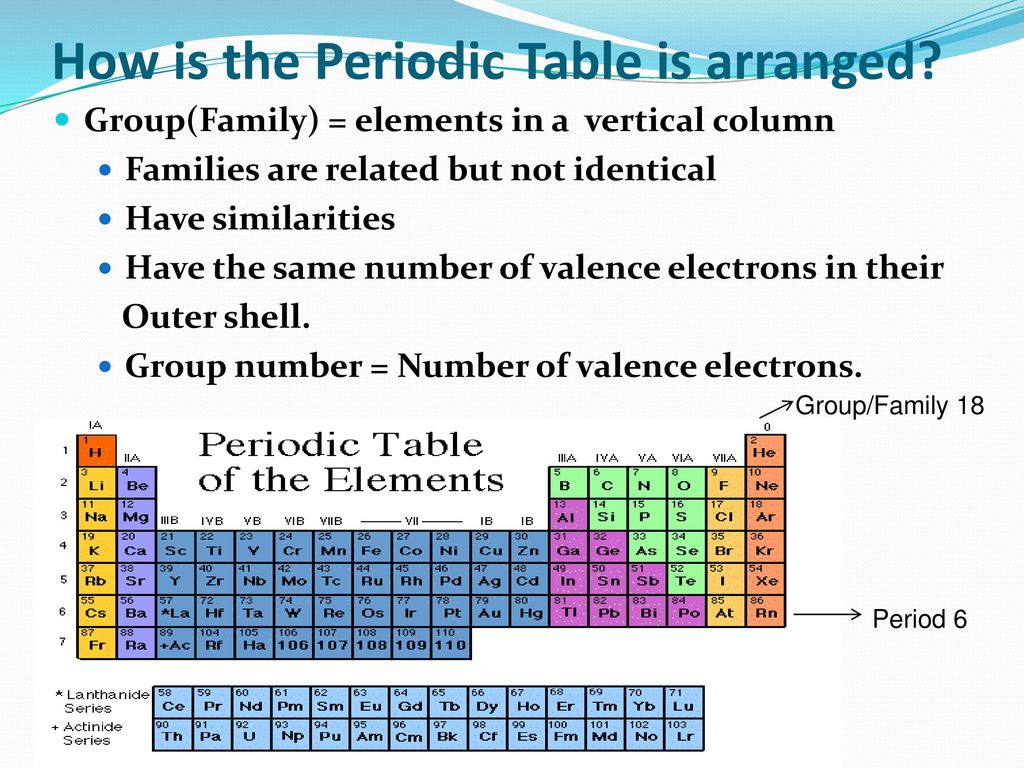
The elements in a group have similarities in the electronic configuration of their atoms and thus they exhibit somewhat related physical and chemical properties. Group in chemistry a set of chemical elements in the same vertical column of the periodic table. It is organized in order of increasing atomic number.
But we virtually ignore the other elements in the centre of the table. In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements. Groups 1 2 except hydrogen and 13 18 are termed main group elements.
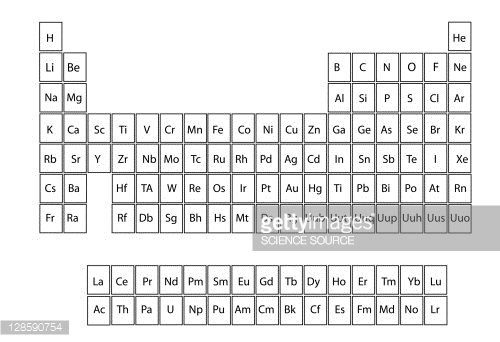
There are only 18 groups in the periodic table that constitute the columns of the table. In the table the elements are placed in the order of their atomic numbers starting with the lowest number of one hydrogen. The periodic table of the chemical elements is a list of known chemical elements.
The group number is an identifier used to describe the column of the standard periodic table in which the element appears. The elements marked with an asterisk in the 2nd column have no stable nuclides. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search.
The periodic table has eight main. Groups 3 12 are termed d block elements.















/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)






/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png)







0 Response to "Periodic Table Group Numbers"
Post a Comment