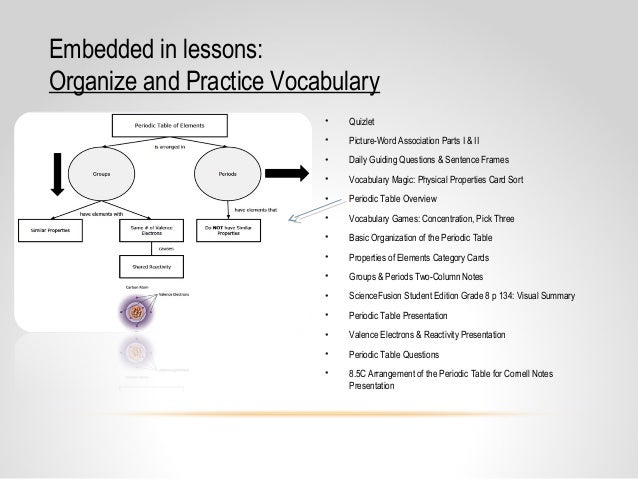
Periodic Table Trends Quizlet
Sorry its super long. Elements in the groups 3a 8a with valence electrons in the s and p orbitals.
The row on the periodic table is the number of these an atom has.

Periodic table trends quizlet. The amount of energy required to remove an electron. The distance from the center of the nucleus to the outermost electrons. Covers the trends discussed in class.
Energy assocaited with loss of 1 electron that. Periodic table trends terms in this set def the energy needed to remove an electron from a neutral gaseous atom. Terms in this set 11 s block.
There are up to 7 of these according to the modern model of the atom. Electrons in lower energy levels block the effective positive charge of the nucleus from valence electrons. Chemistry honors periodic table trends test.
Elements in the transition metals. This review includes the review guide the notes we took and some of the questions from the periodic trend review sheet. Electrons found in the one closest to the nucleus have the lowest energy while those in the outermost one have the highest energy.
Energy associated with gain of desired electron. Increases down the group decreases from left to right. Elements in the first 2 columns with valence electrons in the s orbital.
Chemistry periodic table trends quizlet. Terms in this set 26 atomic radius. Start studying periodic trends.
Measure of how much an atom attracts other electrons towards it. Periodic table trends. Terms in this set 15 electron shielding.
Make sure you have a periodic table with you or you wont be able to answer some of the questions. Learn vocabulary terms and more with flashcards games and other study tools.


























0 Response to "Periodic Table Trends Quizlet"
Post a Comment