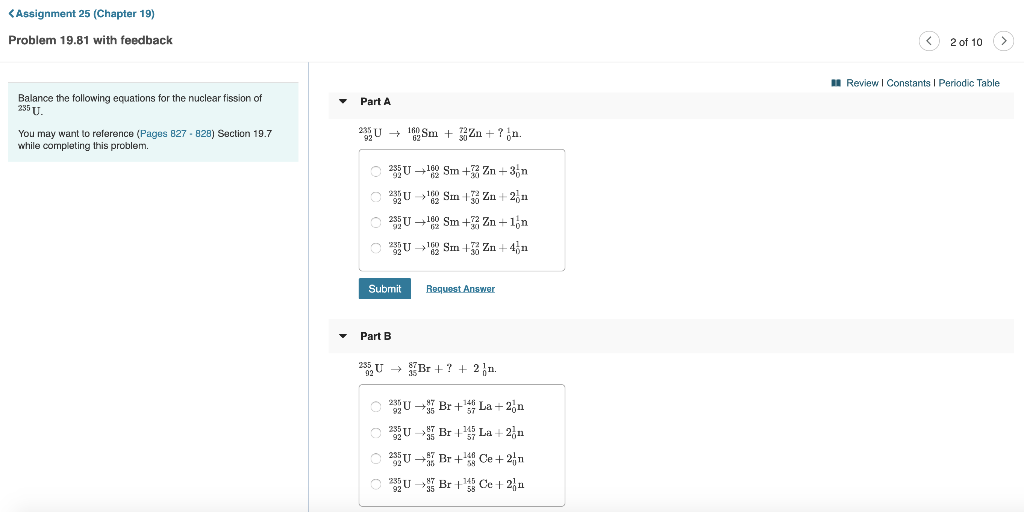
Periodic Table Uranium 235
It has 92 protons and 92 electrons in the atomic structure. Another isotope u 239 is created after the spontaneous fission of u 238.
Naturally occurring uranium consists of 99 uranium 238 and 1 uranium 235.

Periodic table uranium 235. Uranium 235 u is used as a fuel for nuclear power plants as. Uranium fuel used in nuclear reactors is enriched with uranium 235. Uranium is a chemical element with the symbol u and atomic number 92.
Uranium periodic table. The half life of uranium 238 is of 45 billion years while uranium 235 has a half life of only 700 million years. It is a silvery grey metal in the actinide series of the periodic tablea uranium atom has 92 protons and 92 electrons of which 6 are valence electronsuranium is weakly radioactive because all isotopes of uranium are unstable.
Es entsteht durch neutroneneinfang aus 235 u. Definition of uranium 235. Its one of the rarer materials in the earths crust.
In einem kernreaktor erhoht sich der anteil an 236 u deutlich. Humans once thought that uranium was the end of the periodic table. Detailed decay information for the isotope uranium 235 including decay chains and daughter products.
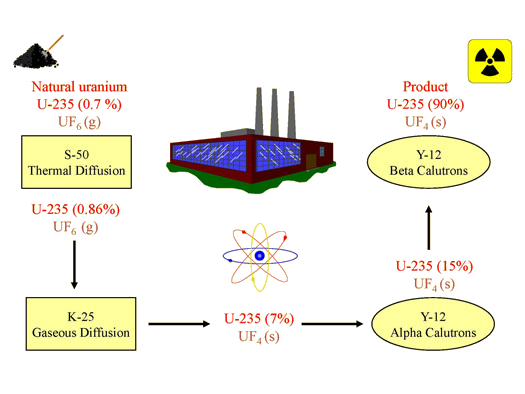
Other two major isotopes of uranium include uranium 235 which is 071 of naturally occurring uranium and uranium 234 00054 of naturally occurring uranium. 235u is a highly strategic fissile material. Uranium 235 is the only naturally occurring fissionable fuel a fuel that can sustain a chain reaction.
Chemical element in the periodic table of elements. The chain reaction is carefully controlled using neutron absorbing materials. Uranium 235 which alone constitutes only 071 of natural uranium is a fissile isotope.
Periodic table uranium 235 which alone constitutes only 071 of natural uranium is a fissile isotope. It is the most stable isotope with half life roughly equal to the age of earth. U 233 and uranium 237 are also formed from u 238 4.
235u is a highly strategic fissile material. Uranium is a 92. Uranium atoms have 92 electrons and the shell structure is 2818322192.
The chemical symbol for uranium is u. Die anteile der isotope 234 u 235 u 236 u in einer urankontamination konnen aufschluss uber deren ursprung geben. Uranium 238 is the most commonly existing isotope of uranium.
The ground state electronic configuration of neutral uranium is rn5f 36d 17s 2 and the term symbol of uranium is 5 l 6. Uranium is a radioactive material that is grey white. The heat generated.
The half lives of its naturally occurring isotopes range between 159200 years and 45 billion years. Description your user agent does not support the html5 audio element. All isotopes of uranium are unstable and radioactive but uranium 238 and uranium 235 have half lives which are sufficiently long to have allowed them to still be present in the solar system and indeed on earth.
Wenn uran einem erhohten neutronenfluss ausgesetzt ist wie z. Its also used in nuclear reactors and nuclear weapons.

































0 Response to "Periodic Table Uranium 235"
Post a Comment