Periodic Table Periods And Families
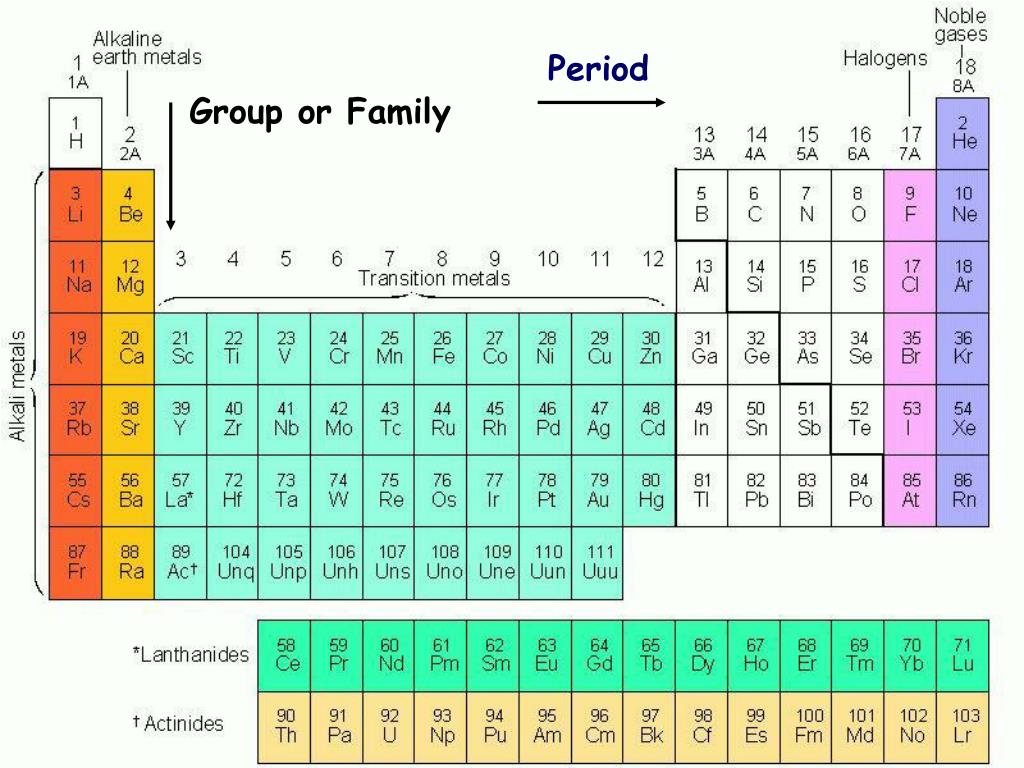
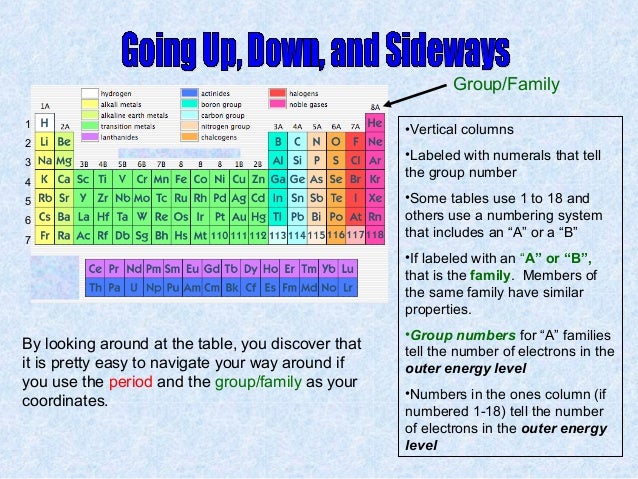
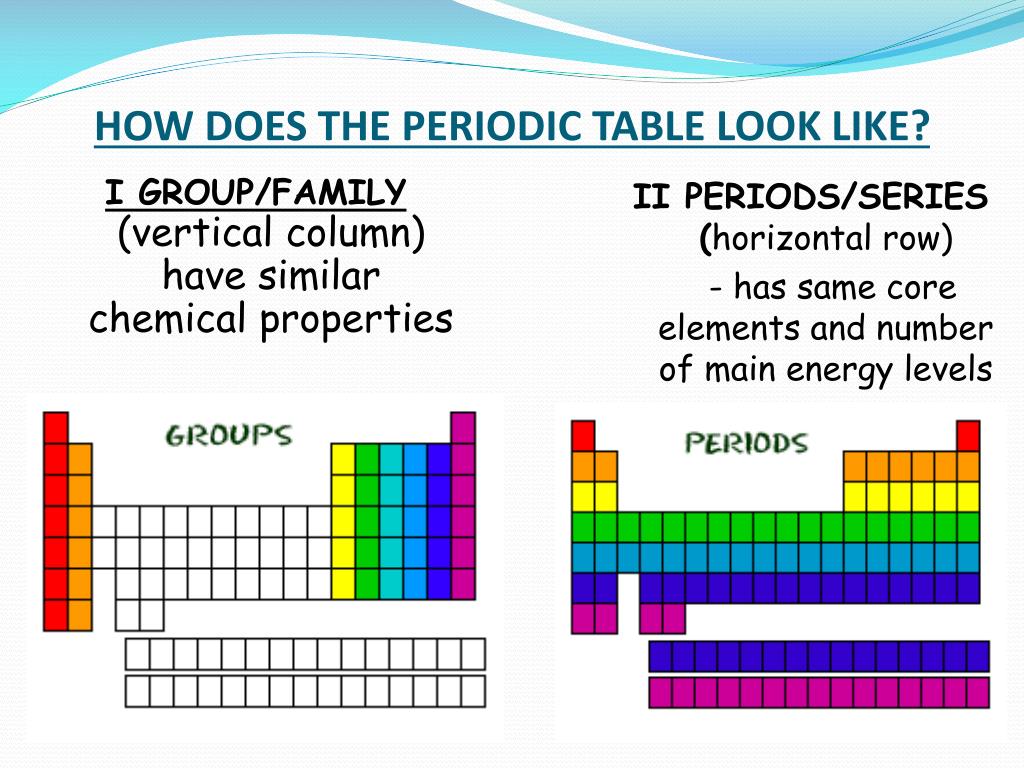
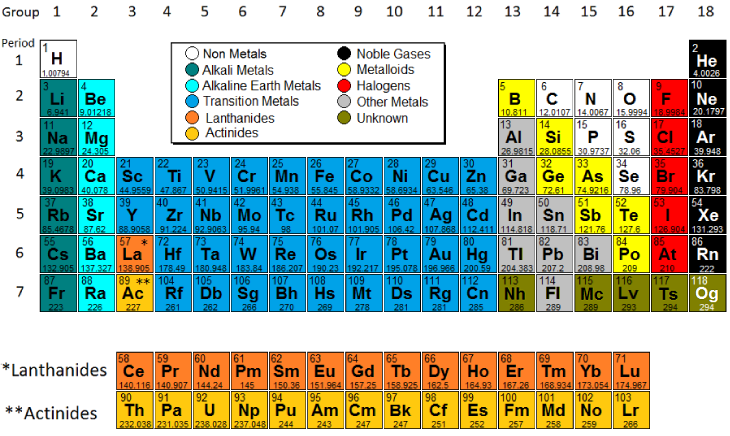
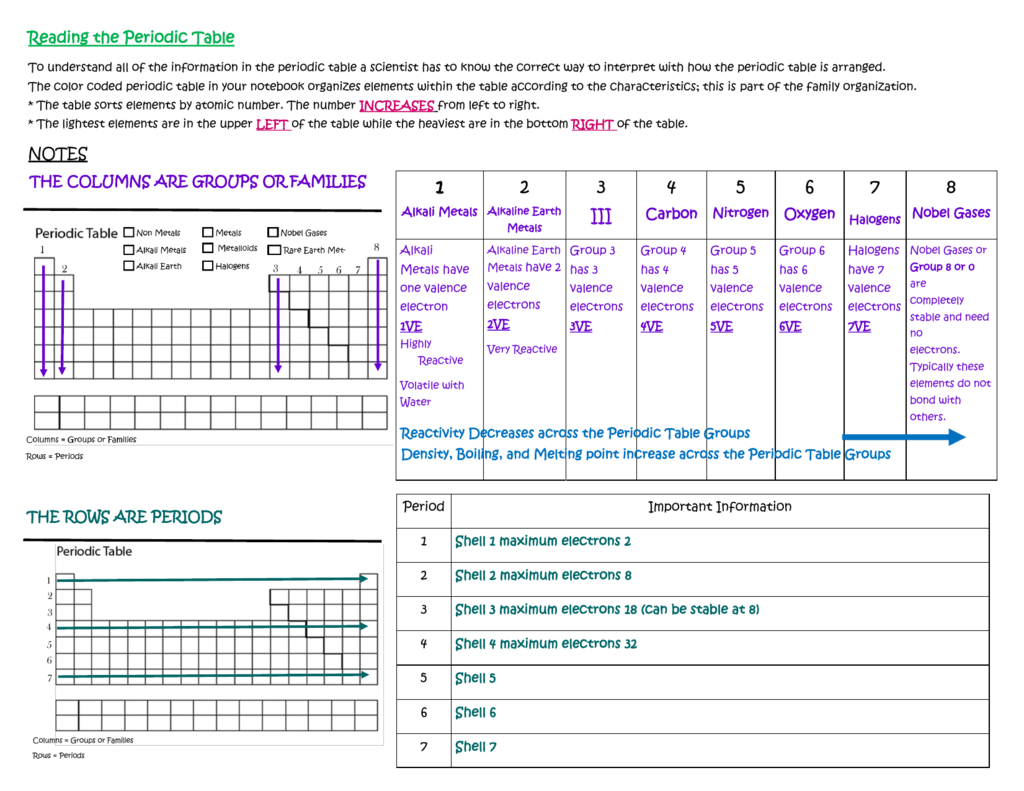
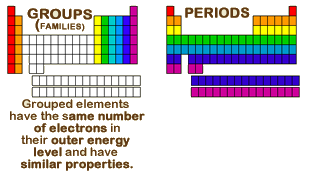
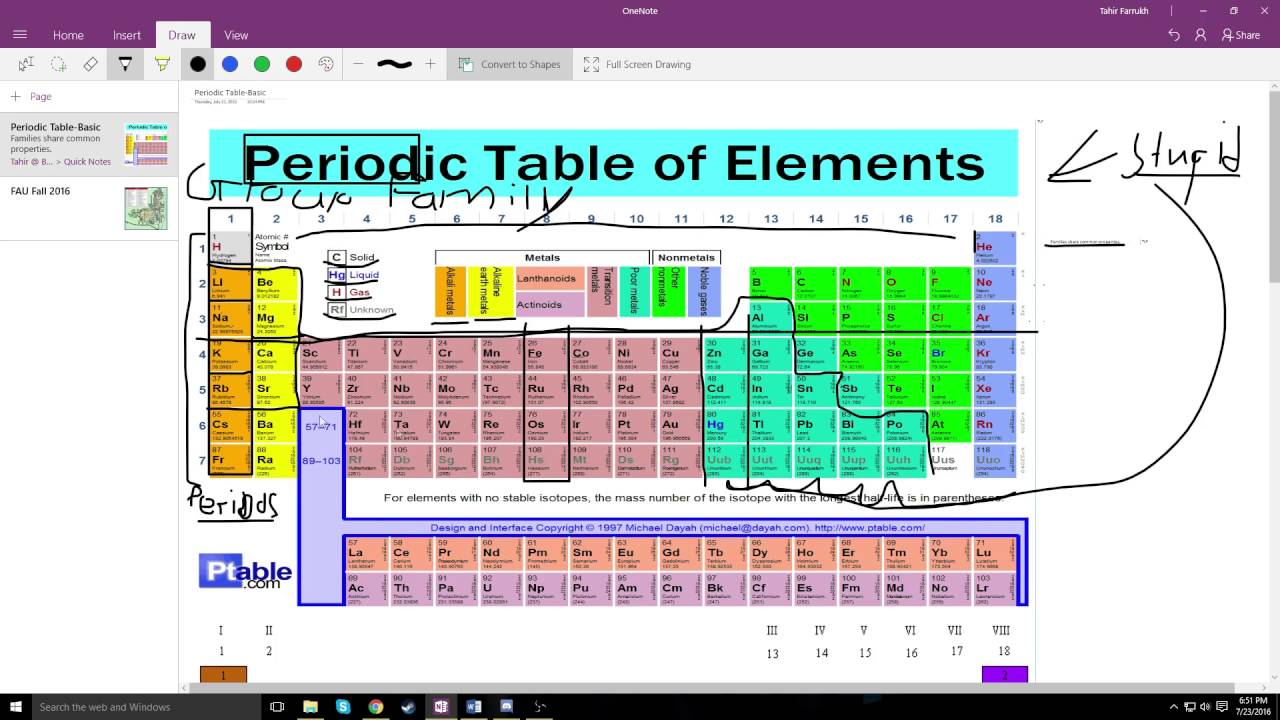
The vertical columns of elements are called groups or families. An element family is a set of elements sharing common properties.
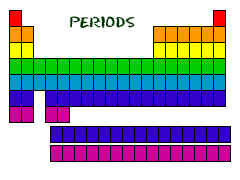
In the periodic table of elements there are seven horizontal rows of elements called periods.

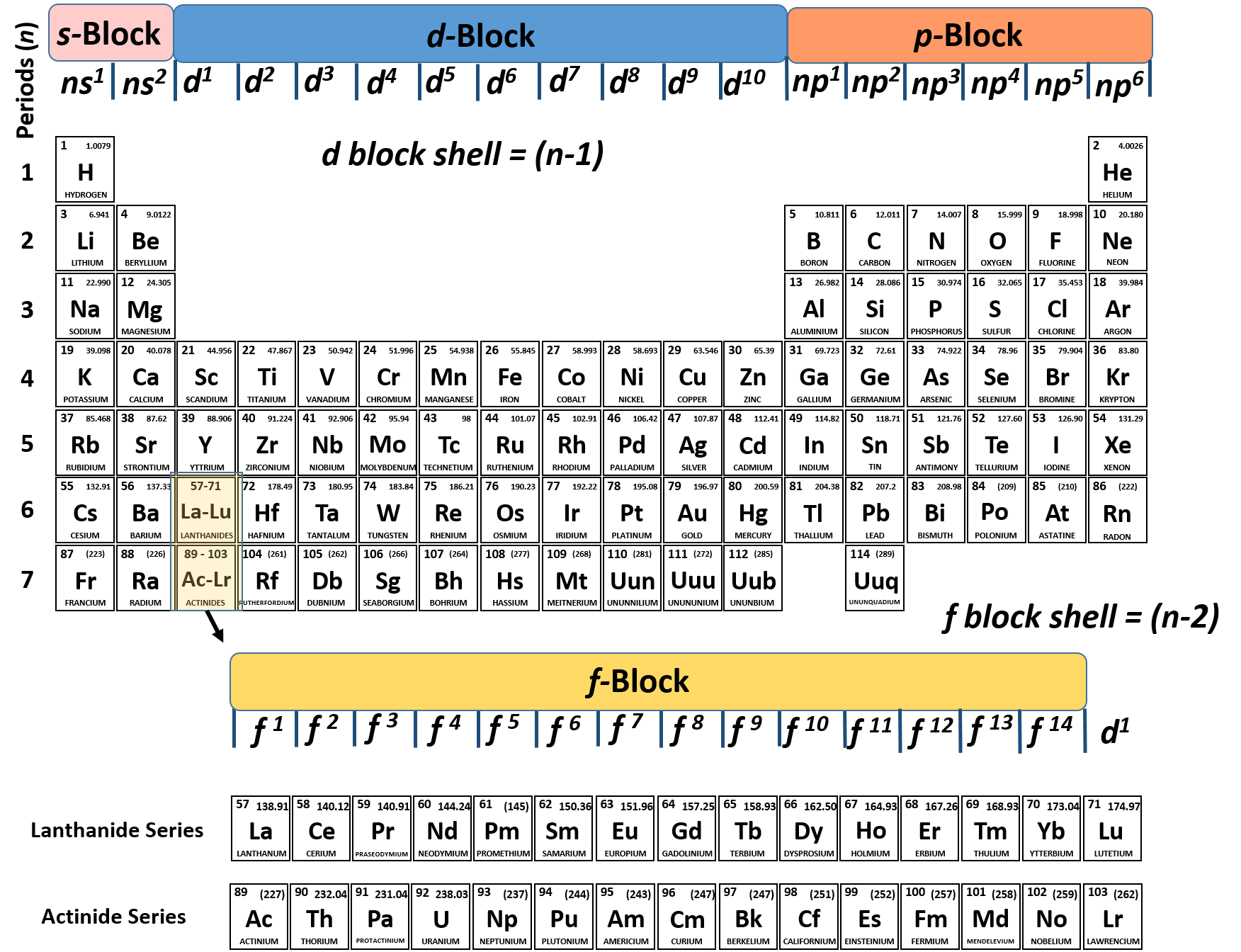
Periodic table periods and families. Families of the periodic table. Elements are classified into families because the three main categories of elements metals nonmetals and semimetals are very broadthe characteristics of the elements in these families are determined primarily by the number of electrons in the outer energy shell. A period in the periodic table is a row of chemical elementsall elements in a row have the same number of electron shellseach next element in a period has one more proton and is less metallic than its predecessor.
The most common way the periodic table is classified by metals nonmetals and metalloids. Remember that mendeleev arranged the periodic table so that elements with the most similar properties were placed in the same group. Periodic table periods and families.
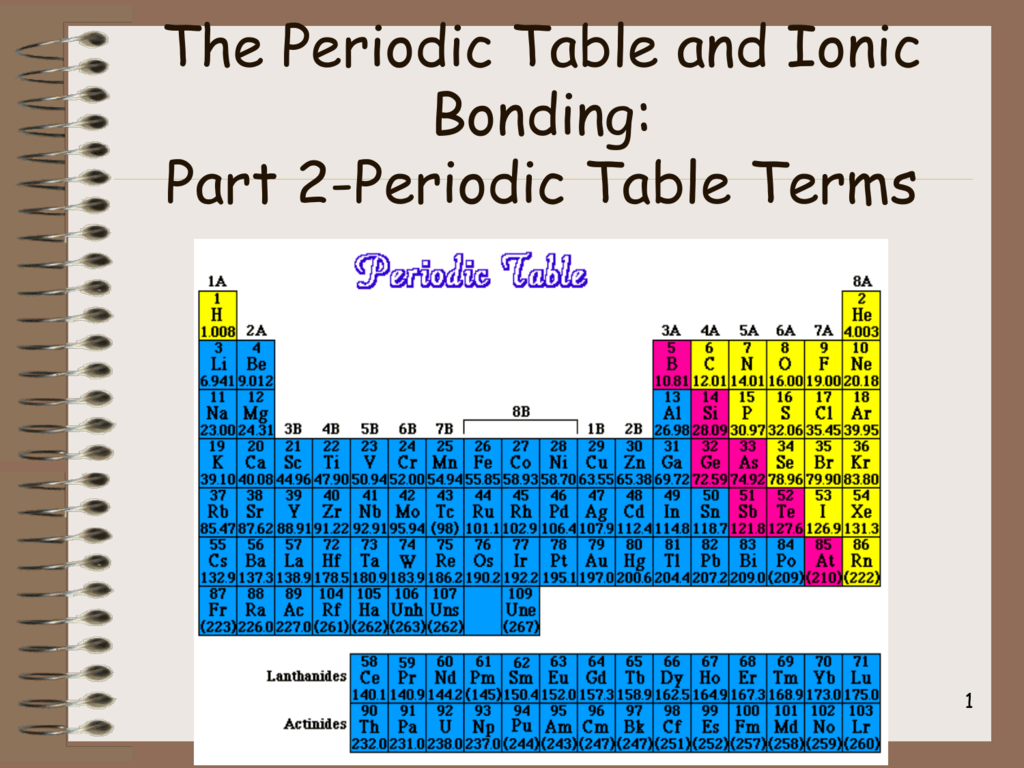
Some of the worksheets for this concept are periodic table work introducing the periodic table periodic table work 2 unit 3 notes periodic table notes anorganizedtablework due theperiodictableof periodic table of elements work elements work name list the basic. Full descriptions from write up sources. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesthe structure of the table shows periodic trendsthe seven rows of the table called periods generally have metals on the left and non metals on the right.
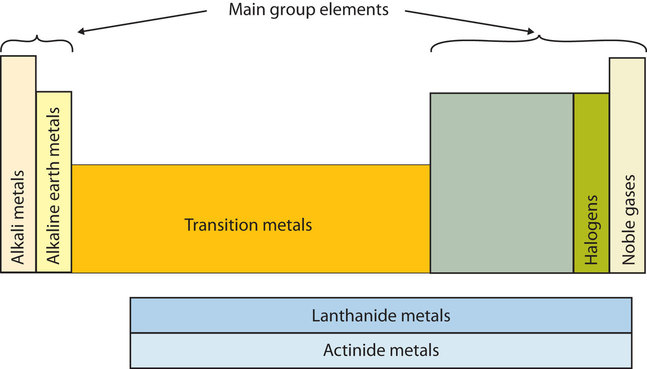
The periodic table can be divided into nine families of elements each having similar properties. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search. Periodic table periods and families.
For example when you go across the table from carbon to nitrogen to oxygen the number of valence electrons increases from 4 to 5 to 6. Periods in the periodic table in each period horizontal row the atomic numbers increase from left. Alkali metals group 1 of the periodic table are the alkali metals.
All of the 1a elements have one valence electron. They have just one electron in their outer shell and they are ready to lose it in ionic bonding. Worksheets are periodic table work introducing the periodic table periodic table work 2 unit 3 notes periodic table notes anorganizedtablework due theperiodictableof periodic table of elements work elements work name list the basic properties of the major.
Displaying all worksheets related to periodic table periods and families. They are highly reactive and do not occur freely in nature. A group is a vertical column of the periodic table.
Periodic table periods and families displaying top 8 worksheets found for this concept. As we go from fluorine to neon to sodium the number of valence electrons increases from 7 to 8 and then drops down to 1 when we start the new period. This is what causes these elements to react in the same ways as the other.
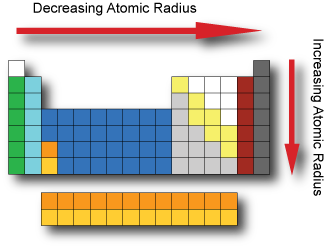
Arranged this way groups of elements in the same column have similar chemical and physical properties reflecting the periodic lawfor example the halogens lie in the second last.


:max_bytes(150000):strip_icc()/periodic-table-165930186-590f2d703df78c92832fe141.jpg)





:max_bytes(150000):strip_icc()/GettyImages-470784875-e7ea7f0da3d74a639d8e6a290afa000b.jpg)


/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)







:max_bytes(150000):strip_icc()/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)



0 Response to "Periodic Table Periods And Families"
Post a Comment