Periodic Table Of Elements Carbon Family
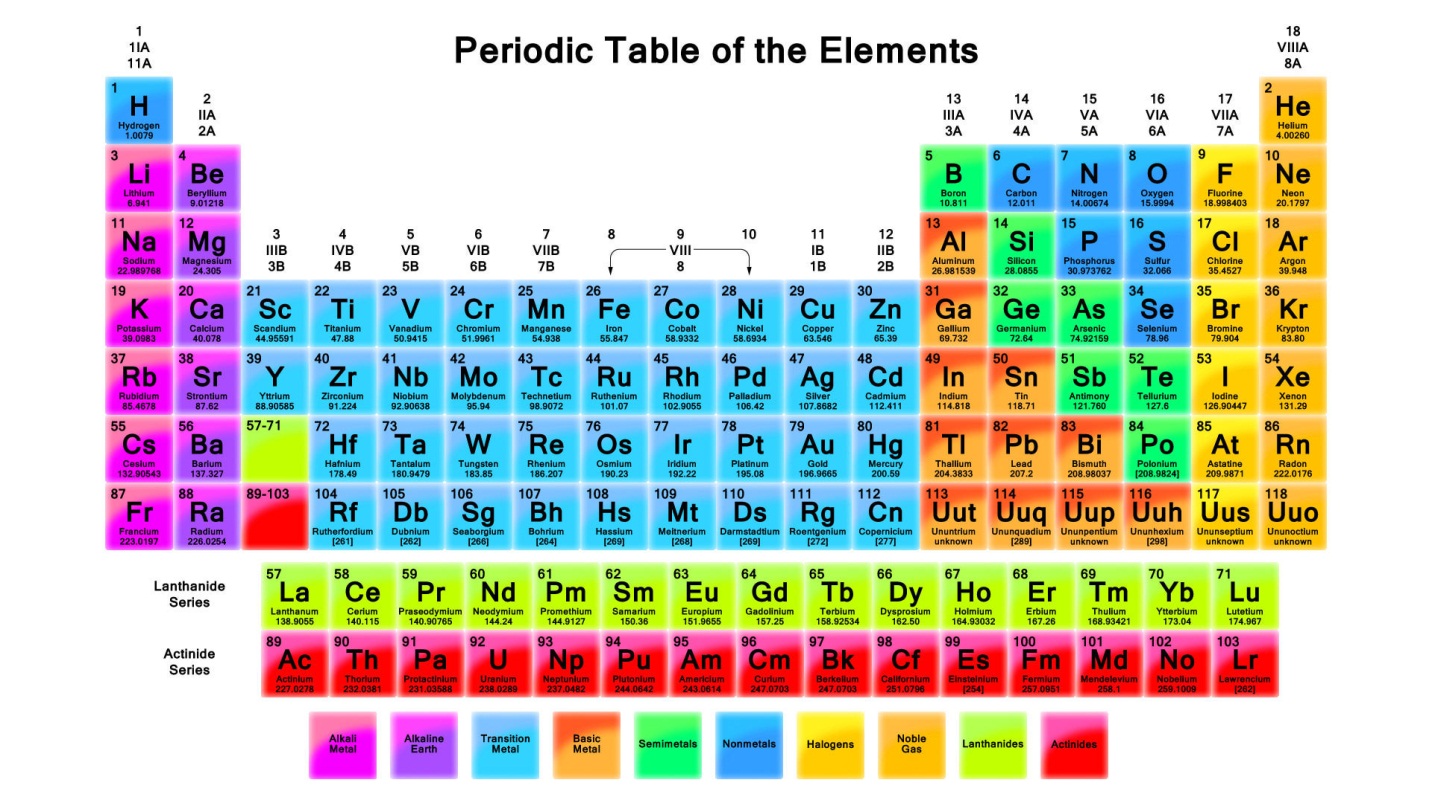
The carbon family is located very nearly in the middle of the periodic table with nonmetals to its right and metals to its left. It lies within the p block.
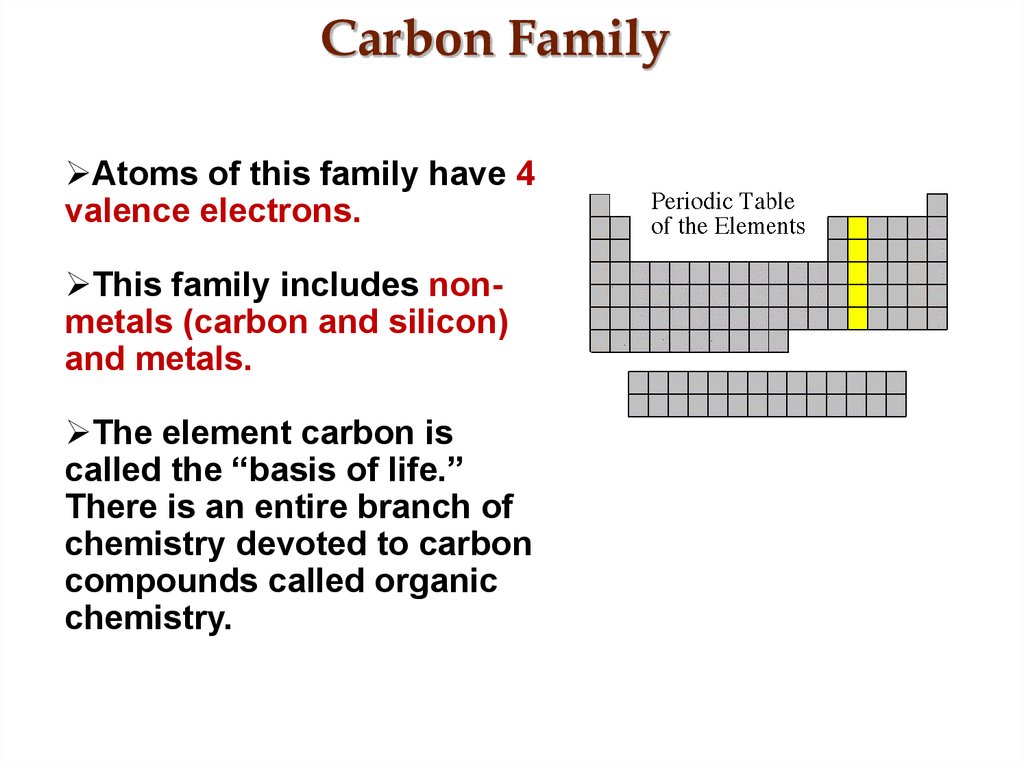
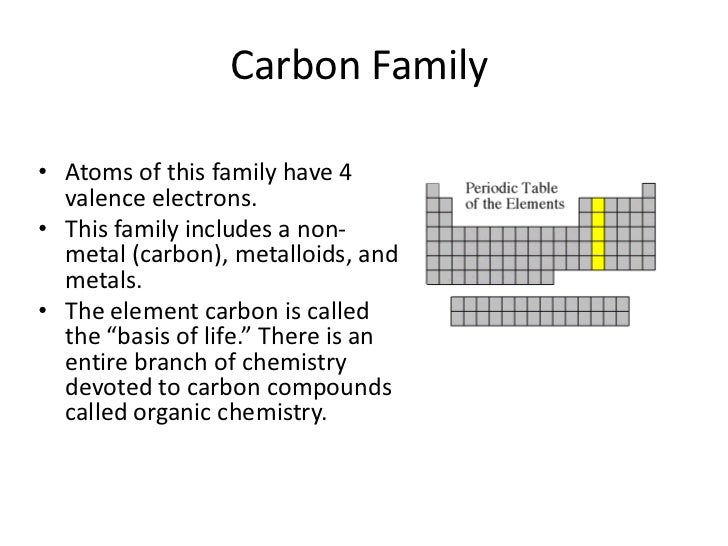
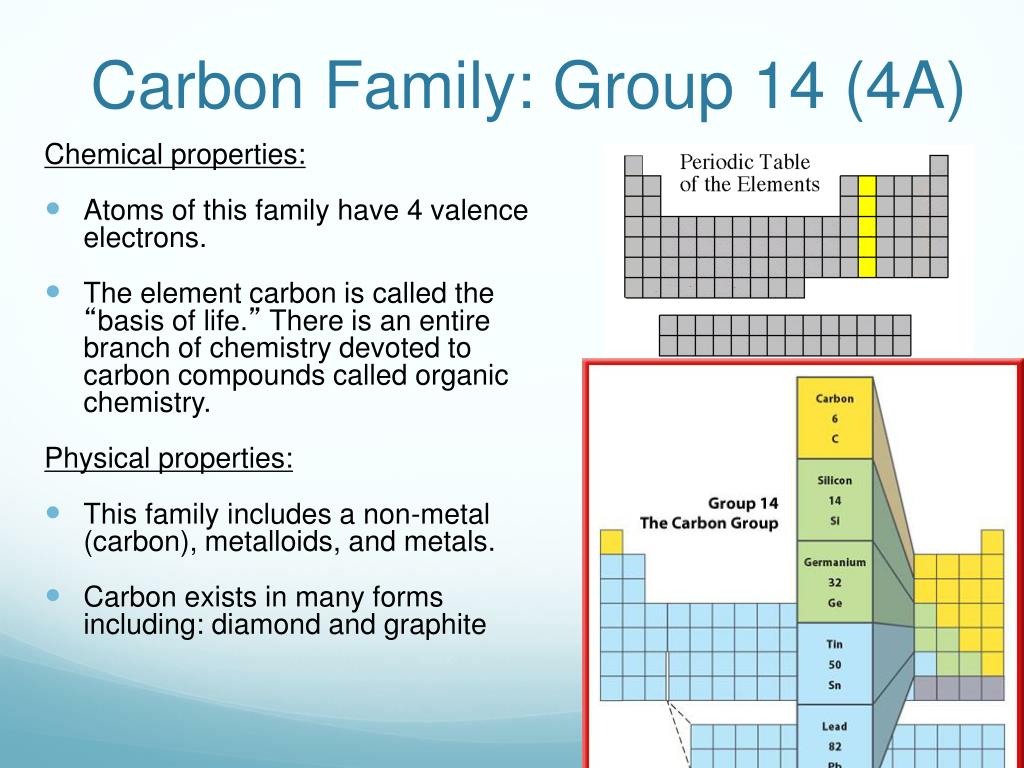
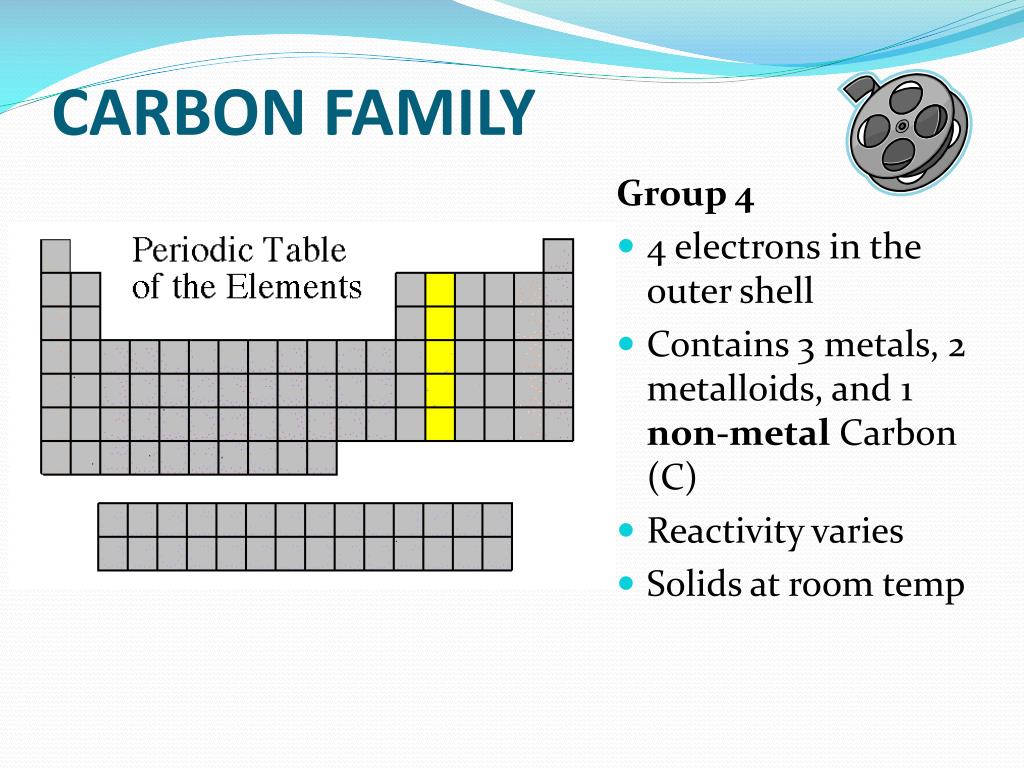
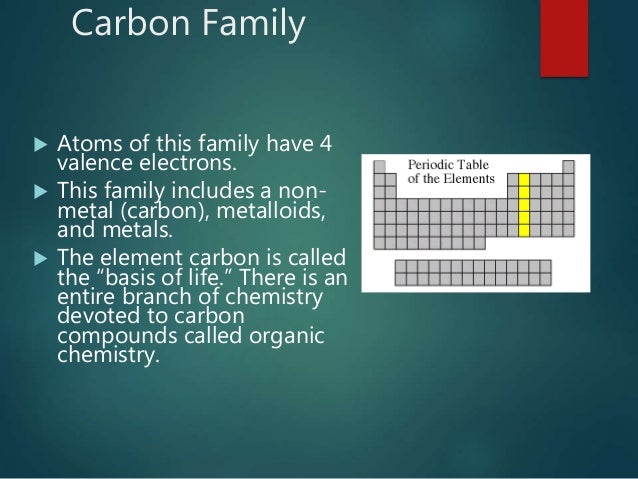
It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bonds.

Periodic table of elements carbon family. Carbon is an element in the non metals group. It is found in abundance in the sun stars comets and atmospheres of most planets. The carbon family is group 14.
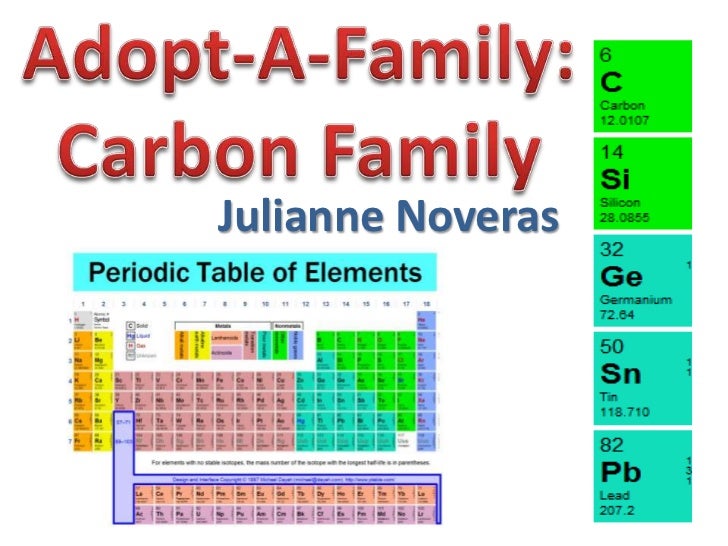
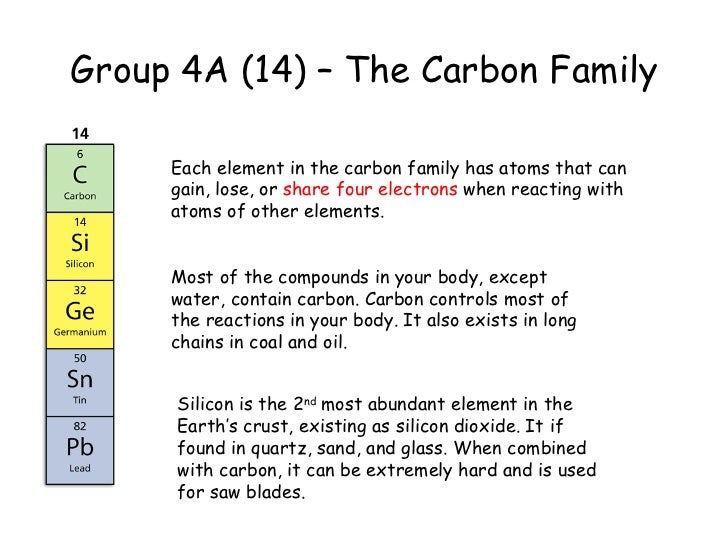
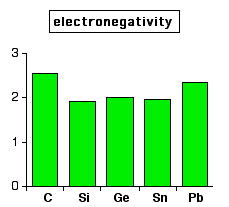
The carbon family consists of five elements. We can thus know their electronic configuration is ns 2 np 2. The carbon group is a periodic table group consisting of carbon c silicon si germanium ge tin sn lead pb and flerovium fl.
It is a component of rocks as carbonates of calcium limestone magnesium and iron. Elements are classified into families because the three main categories of elements metals nonmetals and semimetals are very broadthe characteristics of the elements in these families are determined primarily by the number of electrons in the outer energy shell. Carbon group element any of the six chemical elements that make up group 14 iva of the periodic tablenamely carbon c silicon si germanium ge tin sn lead pb and flerovium fl.
The carbon family is element group 14 of the periodic table. It is likely that element 114 flerovium will also behave in some respects as a member of the family. We can find the carbon family towards the right side of the periodic table.
We refer to them as the group 14 elements. The periodic table can be divided into nine families of elements each having similar properties. An element family is a set of elements sharing common properties.
Carbon silicon germanium tin and lead. There is no carbon family on the periodic table. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search.
These elements belong to the p block of elements in the periodic table. Periodic table modern version of the periodic table of the elements. Full descriptions from write up sources.
In modern iupac notation it is called group 14. In other words the group consists of carbon and the elements directly below it on the periodic table. Carbon is a chemical element with symbol c and atomic number 6.
It belongs to group 14 of the periodic table. They have just one electron in their outer shell and they are ready to lose it in ionic bonding. Carbon is a group 14 element and is distributed very widely in nature.
They are highly reactive and do not occur freely in nature. Alkali metals group 1 of the periodic table are the alkali metals. The members of this family include carbon c silicon si germanium ge tin sn lead pb and flerovium fl.
Carbon is present as carbon dioxide in the atmosphere and dissolved in all natural waters.
























0 Response to "Periodic Table Of Elements Carbon Family"
Post a Comment