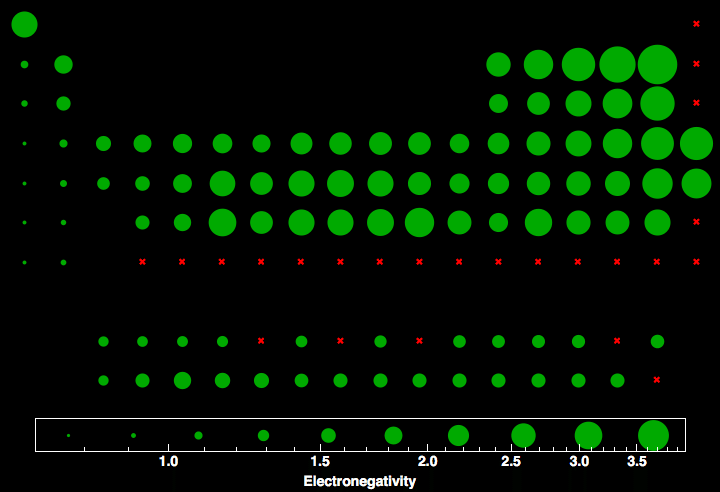
Periodic Table Electronegativity Graph
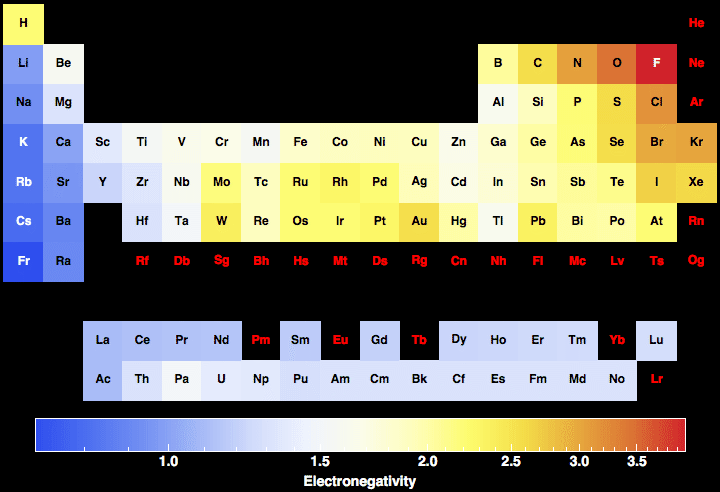
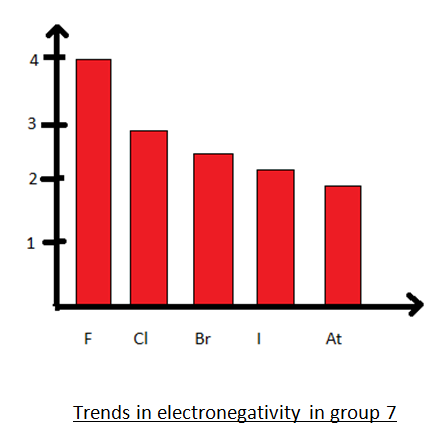
In the below periodic table you can see the trend of electronegativity. Generally electronegativity increases from left to right and decreases as you move down a group.
Click on each element to explore atomic view.
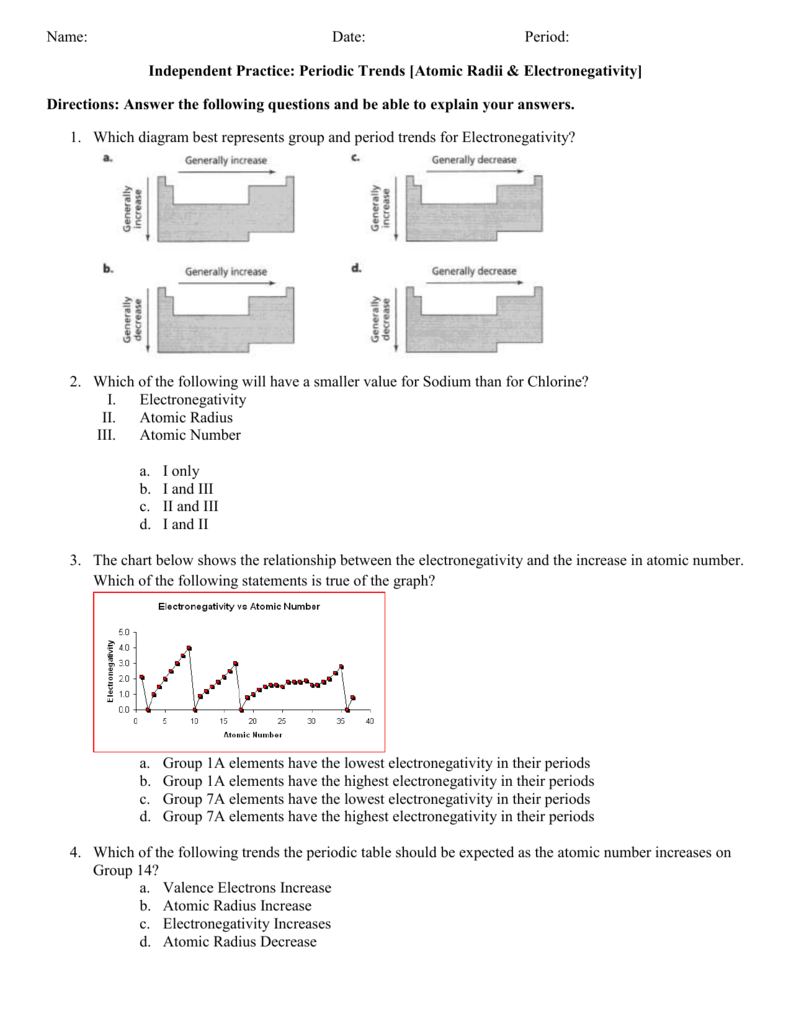
Periodic table electronegativity graph. This later version may be more useful and easier to review than the periodic table itself if we are just interested in checking out the electronegativity value of each of the elements and nothing else. It is not completely correct but has many features that are sufficient for a general understanding. This model was proposed by niels bohr in 1913.
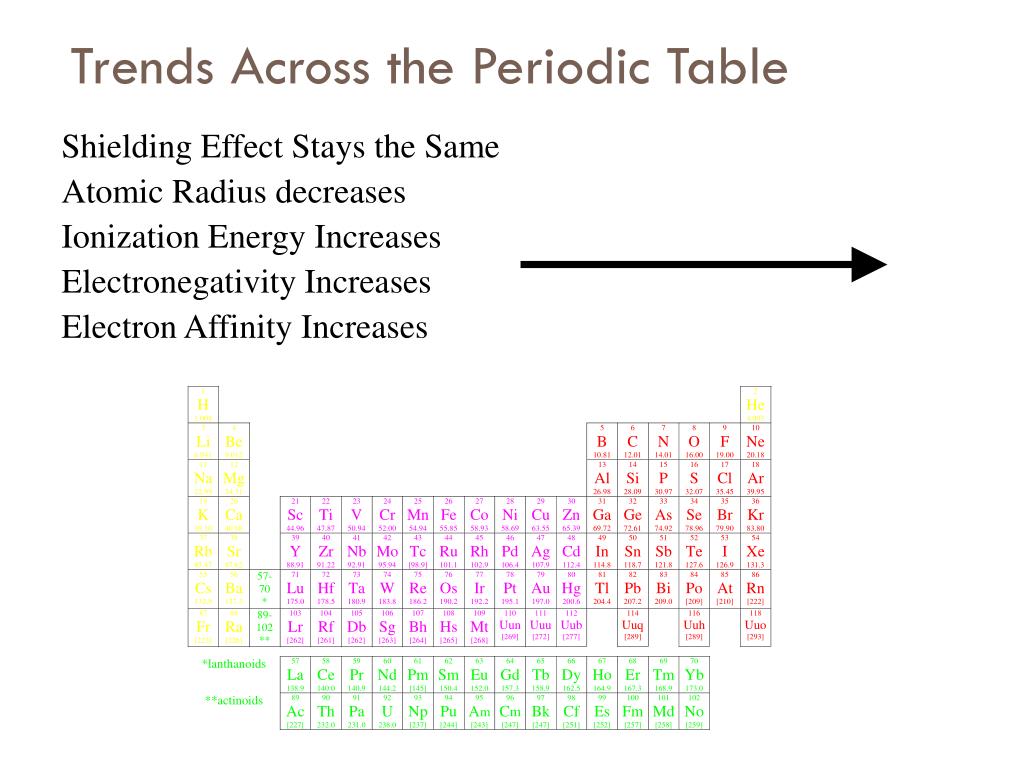
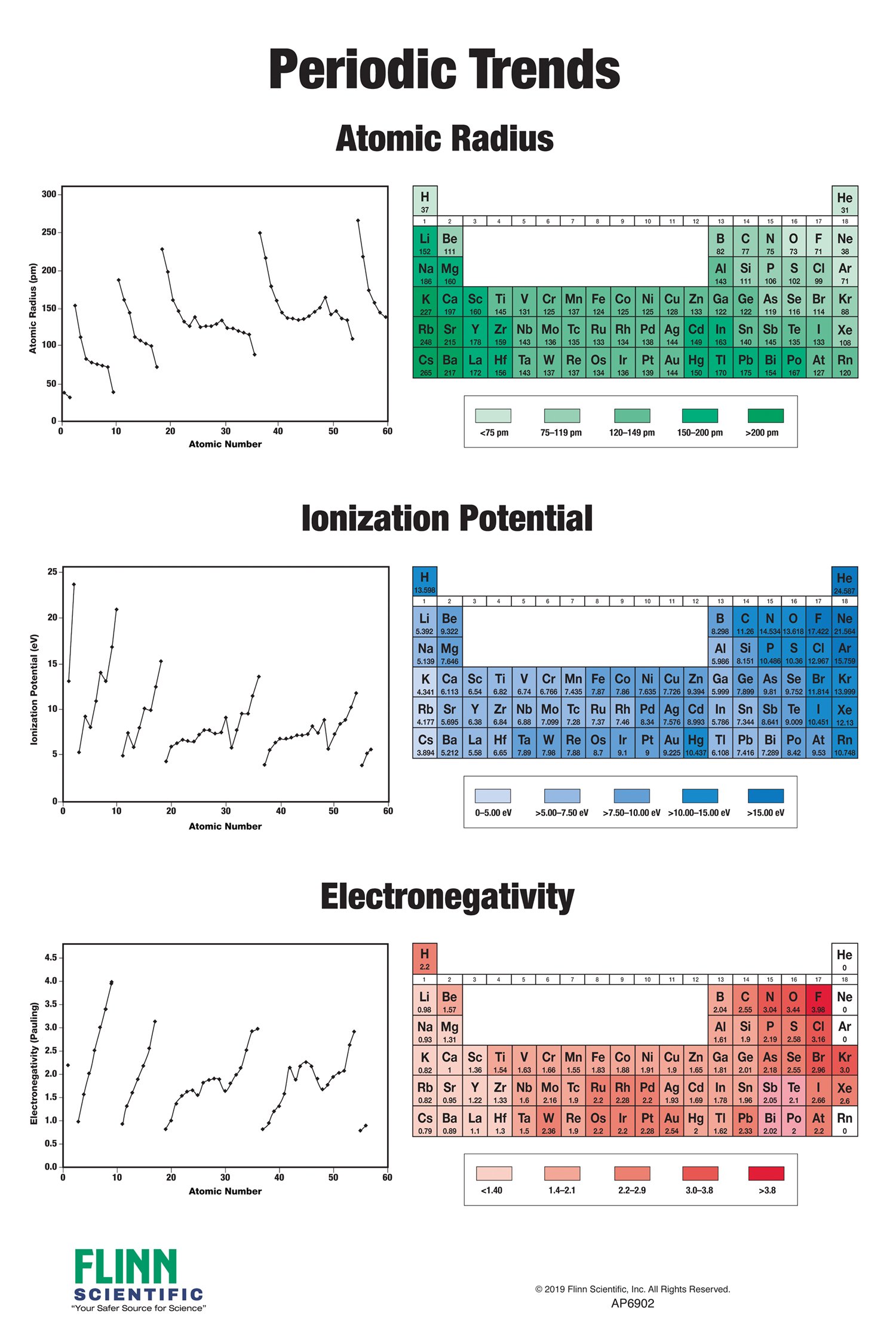
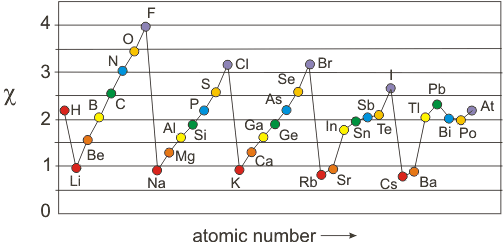
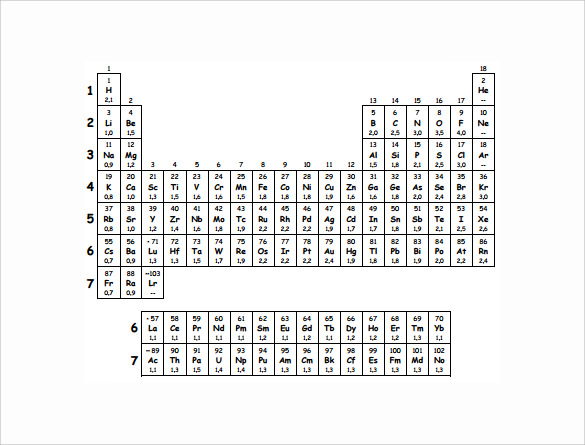
The table gives values on the pauling scale which have no units. Fluorine 398 is the most electronegative element. When one moves left to right in the periodic table the atomic radius of the elements decreases ionization energy increases and electronegativity increases.
Keep in mind the noble gasses column at the right hand side of the periodic table are relatively inert so their electronegativity approaches zero exception to the overall trend. There are particular electronegativity trends that are seen in the periodic table. This 3d representation has a table view which shows initially and an atomic view.
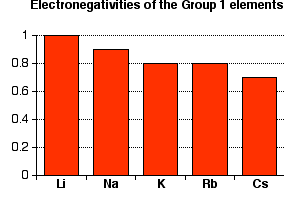
Click on a column or row icon to see how the electronegativity varies within that column or row. Electronegativity reflects how easily an atom can form a chemical bond. When one moves from top to bottom the atomic radius increases the ionization energy decreases and the electronegativity too decreases.
Point to the graph to see details or click for full data on that element. Click here to buy a book photographic periodic table poster card deck or 3d print based on the images you see here. Nevertheless the ionization increases.
Keep in mind that electronegativities are approximate measures of the relative tendencies of these elements to attract electrons to themselves in a chemical bond. This is a bohr model of the atom. A 3d visualization of periodic table.
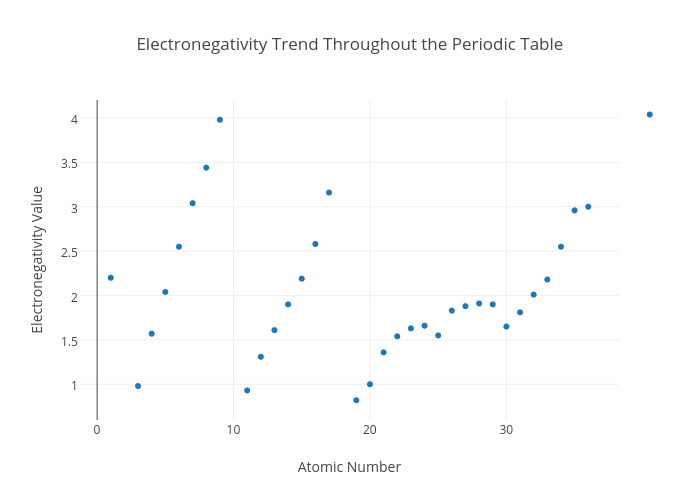
Electronegativity is the ability of an atom to attract the bonding electrons in a covalent bond. Periodic table of elements with electronegativity trends. The electronegativity chart can be organized in the same order as the periodic table but it can also be rearranged so that it shows the elements according to their electronegativity value.
For facts physical properties chemical properties structure and atomic properties of the specific element click on the element symbol in the below periodic table. The larger the difference between electronegativity values the more likely two atoms are to form a chemical bond. The following periodic table shows the pauling electronegativity data for the elements.
When you take a closer look at a periodic table that includes the different electronegativity numbers for each specific element of the periodic table youll notice that when you are moving from the left to the right the atomic radius of the elements decreases. Periodic table of elements. Up to date curated data provided by mathematica s elementdata function from wolfram research inc.
The greater an atoms electronegativity the greater its ability to attract electrons to itself. Periodic table with electronegativity.




/PeriodicTableEnegativity-56a12c955f9b58b7d0bcc69d.png)







:max_bytes(150000):strip_icc()/Periodic_variation_of_Pauling_electronegativities-56a12b2f3df78cf772680e68.jpg)










0 Response to "Periodic Table Electronegativity Graph"
Post a Comment