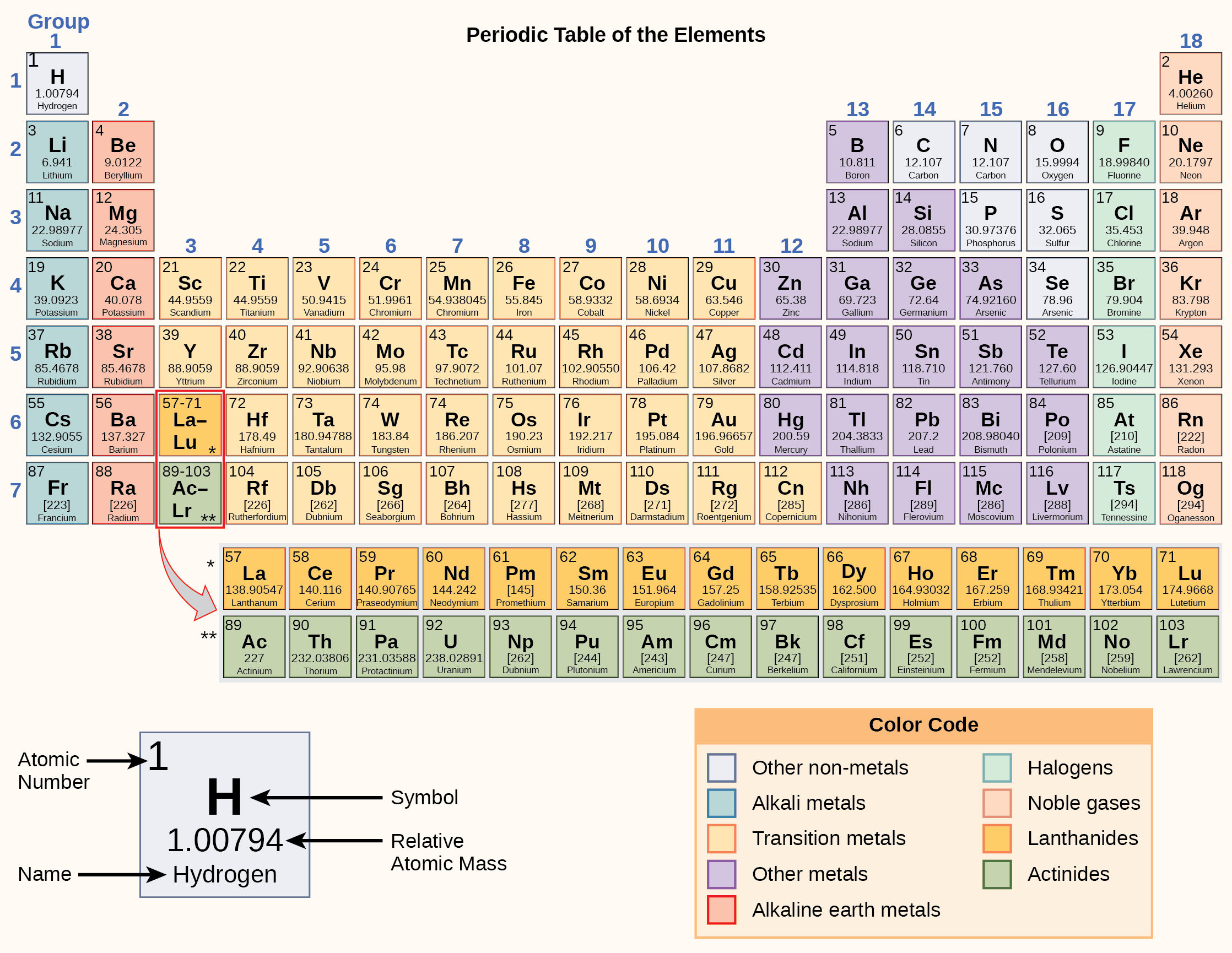
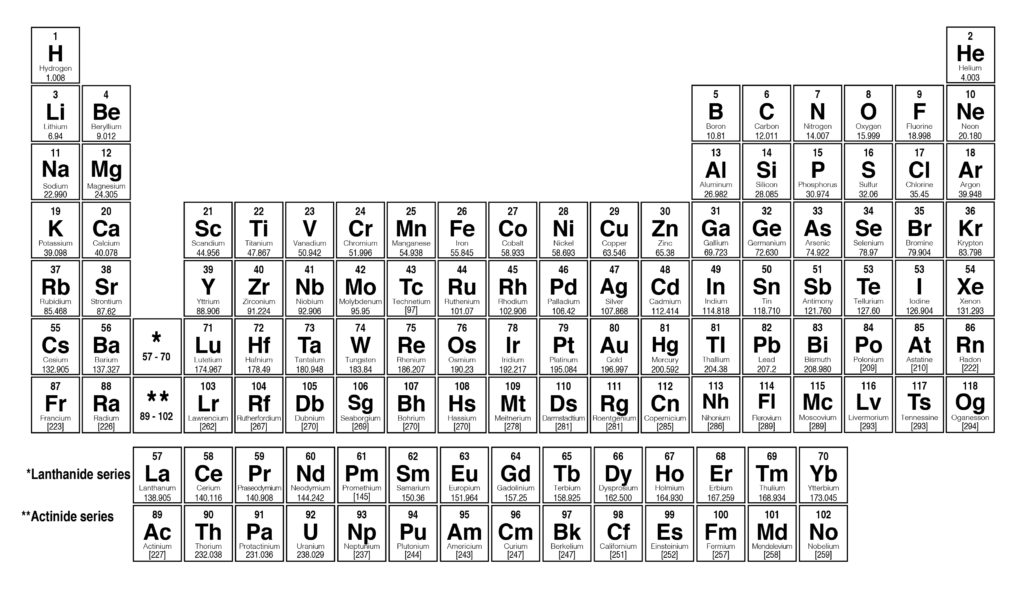
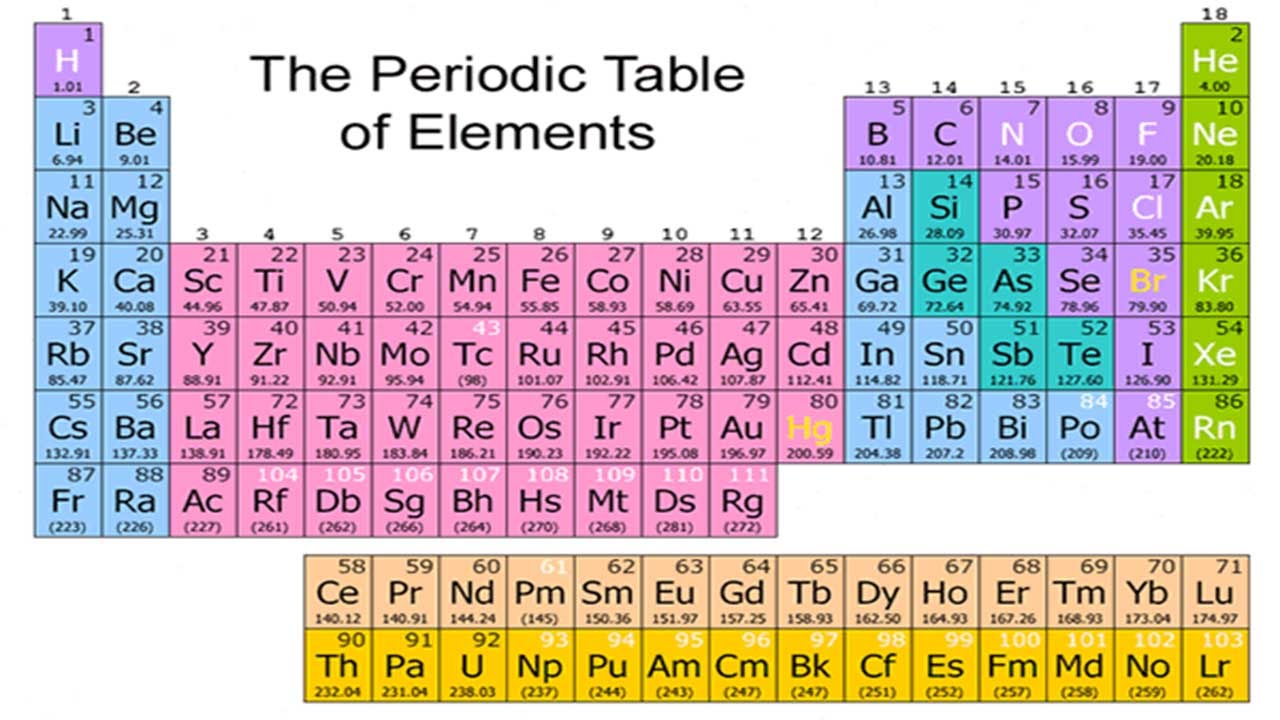
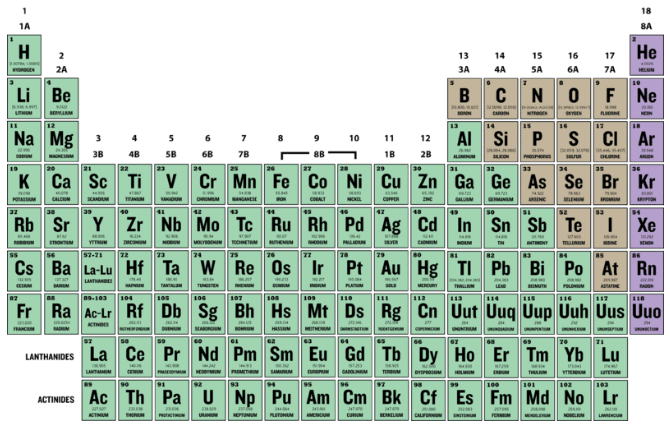
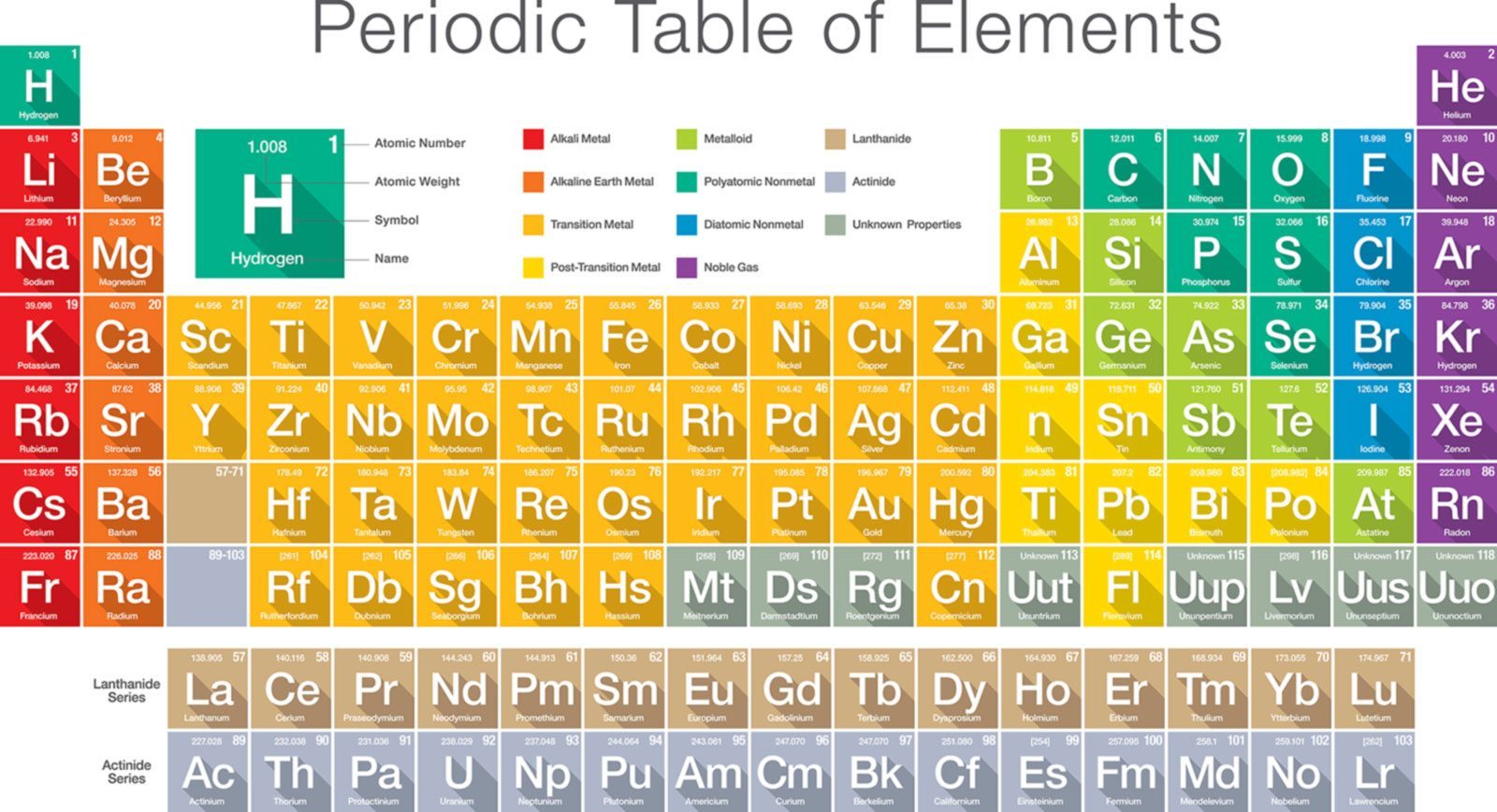
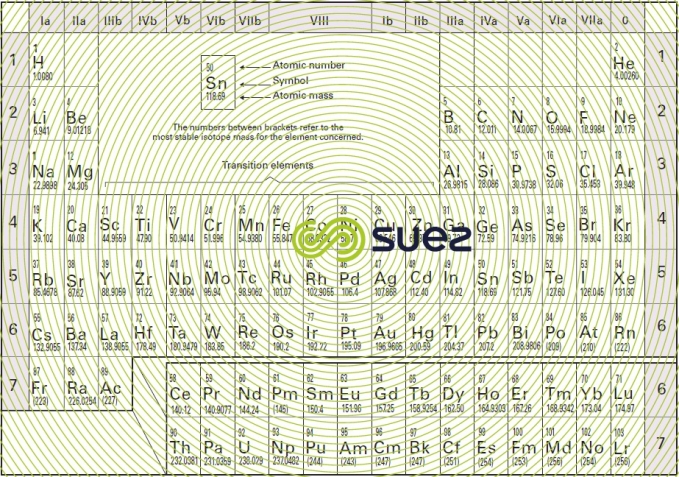
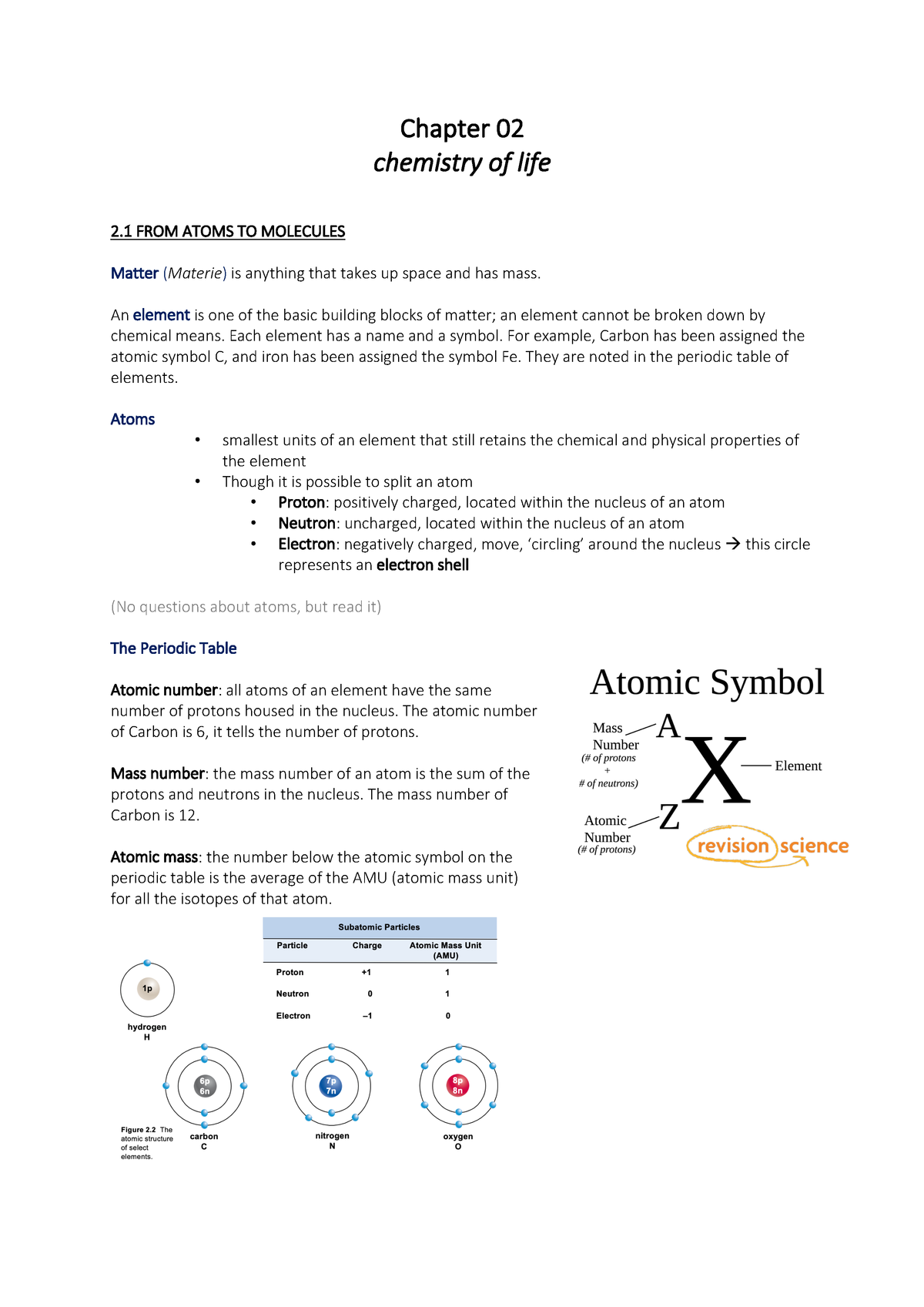
Periodic Table With Atomic Mass And Proton Number
In this table an elements atomic number is indicated above the elemental symbol. Chemical symbol of chlorine 35 the proton number is shown below the chemical symbol and the mass number is shown above.
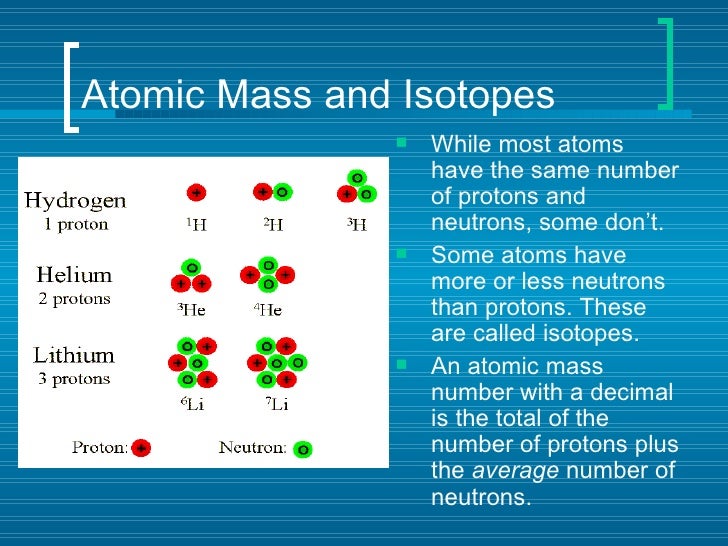
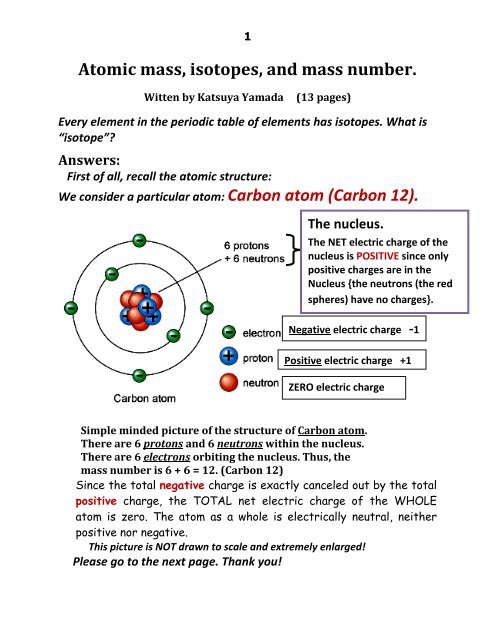
Atomic mass number given for longest lived isotope.
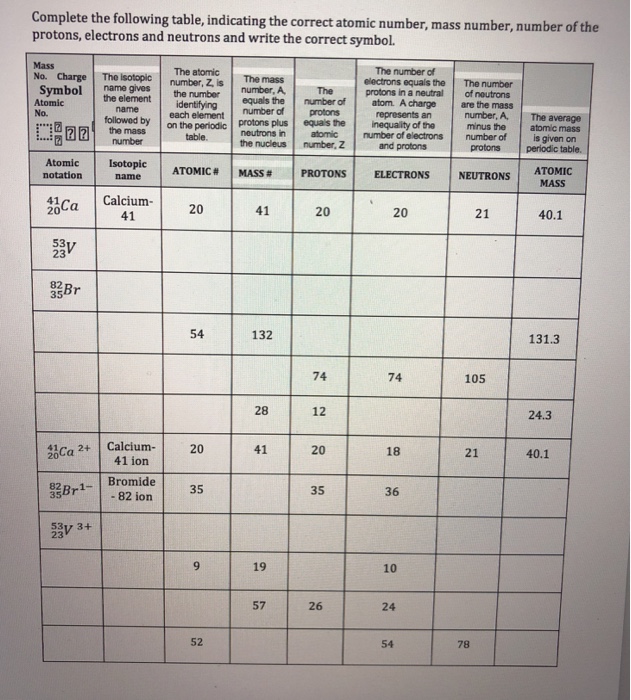
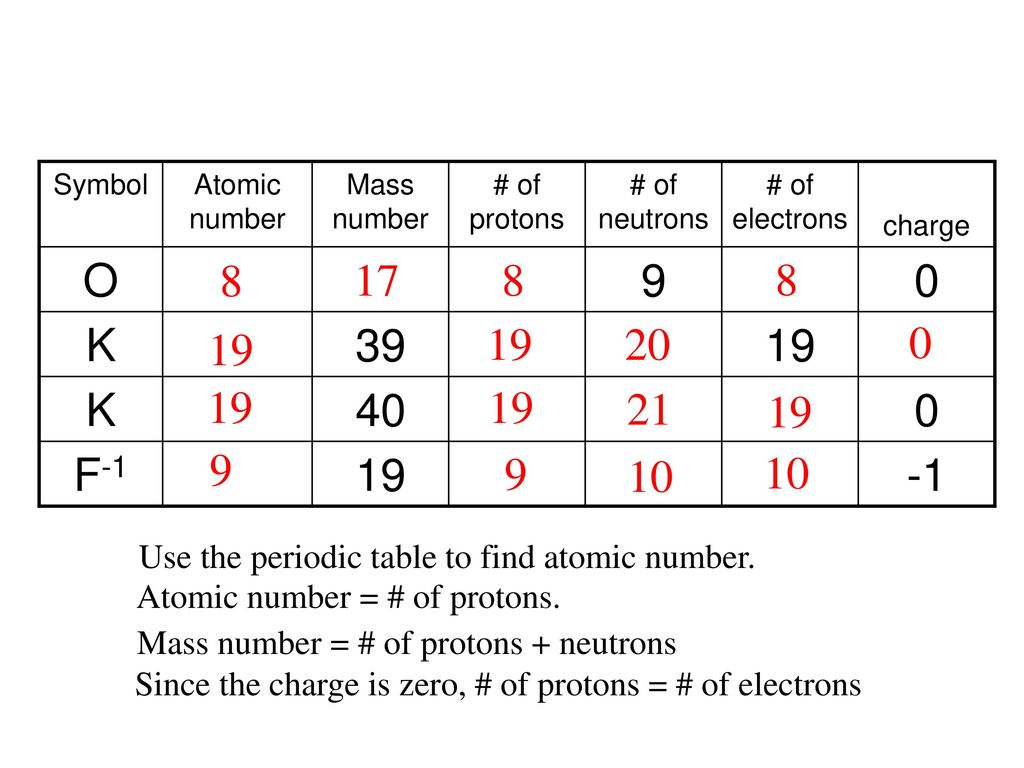
Periodic table with atomic mass and proton number. Atomic mass number given for longest lived isotope. Atomic mass is measured in atomic mass units amu which are scaled relative to carbon 12 c that is taken as a standard element with an atomic mass of 12. Finding the number of protons neutrons and electrons in a given element isnt as hard as it sounds.
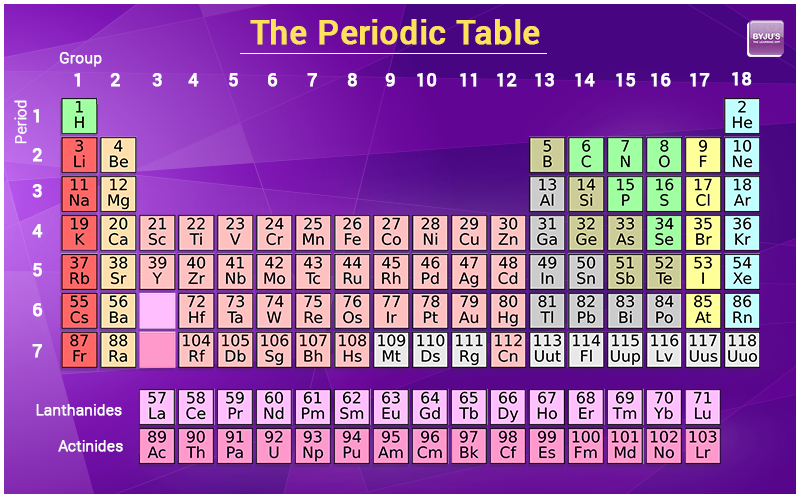
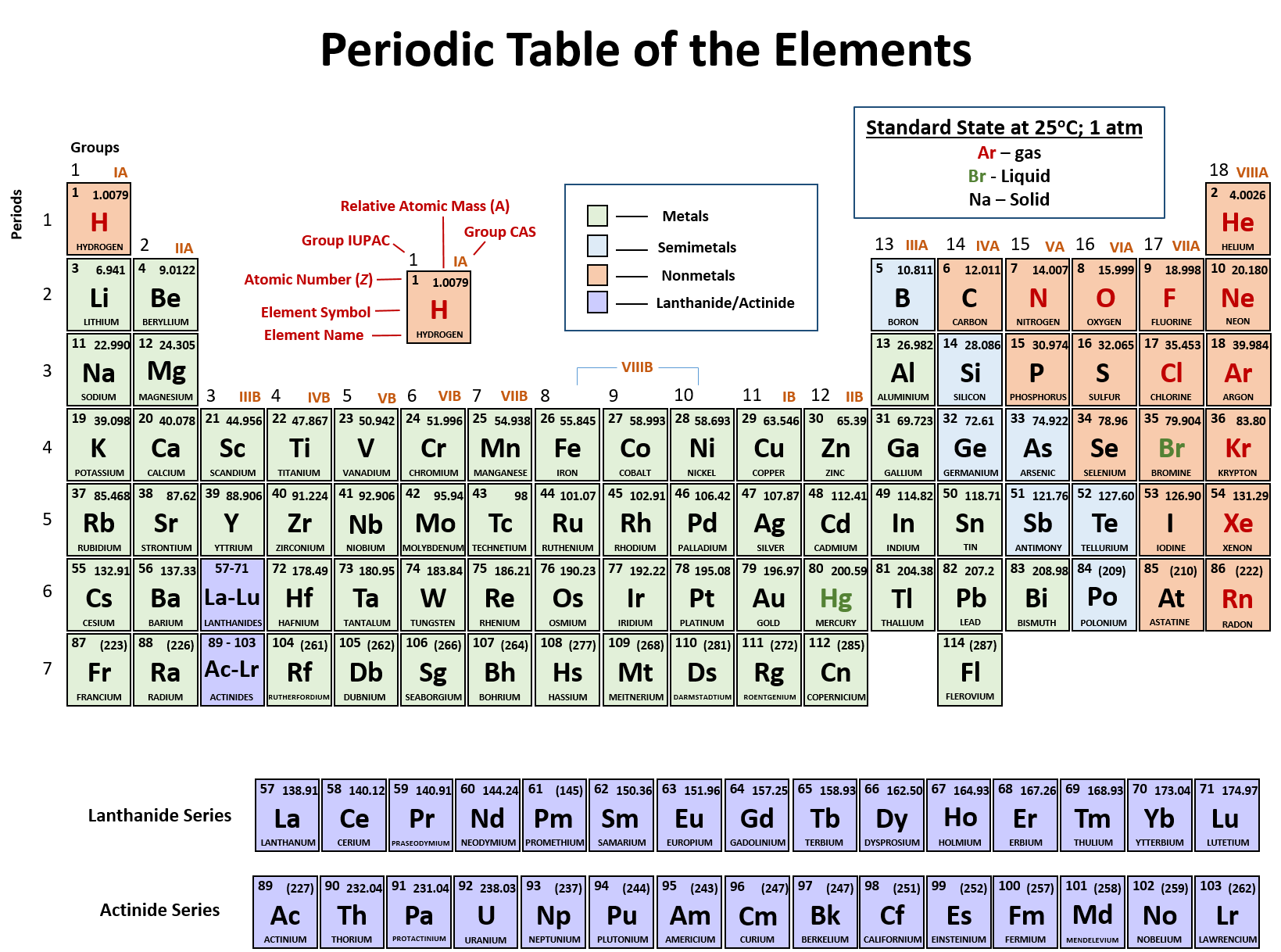
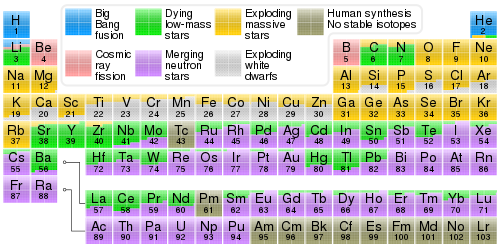
Atoms contain protons neutrons and electrons. The periodic table is a very useful listing of all 118 elements by symbol atomic number and atomic mass and molecular mass. Elements with similar chemical properties are called group.
Notes on the atomic mass of particular elements. The periodic table is a chart of all the elements arranged in increasing atomic number. Atoms contain protons neutrons and electrons.
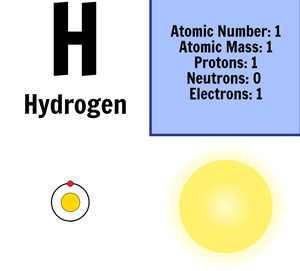
The electrons are arranged in shells around the nucleus. The periodic table is a chart of all the elements arranged in increasing atomic number. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
Lithium atoms have three protons beryllium atoms have four and so on. Hydrogen at the upper left of the table has an atomic number of 1. The periodic table provides us with a comprehensive view of the various elements.
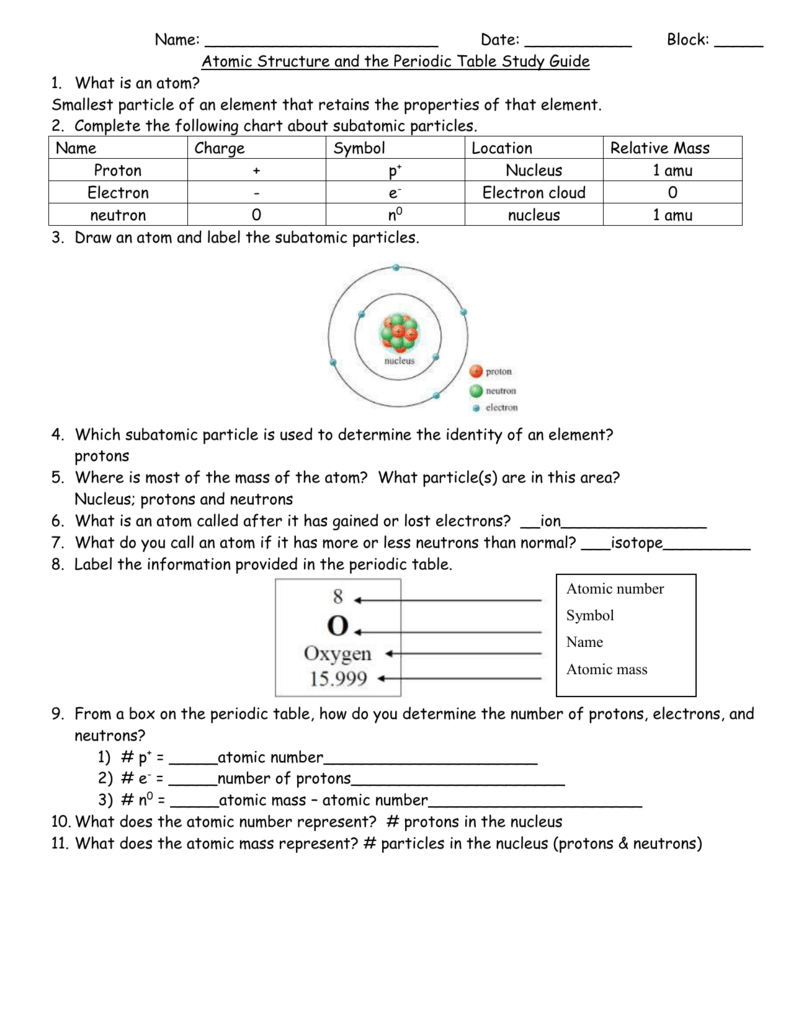
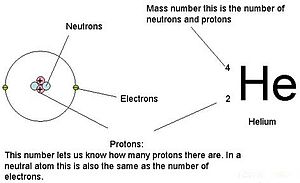
The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structurethe chemical symbol for hydrogen is h. In an atom is called its mass number.
Next on the table is helium whose atoms have two protons in the nucleus. Its monatomic form h is the most abundant chemical substance in the universe constituting roughly 75 of all baryonic mass. Every hydrogen atom has one proton in its nucleus.
The following article will help you to gain more information about the same. Along with atomic mass and atomic number values this table helps us to understand the various properties abbreviations and names of all the elements present in nature. Oftentimes part of your answer will be right in front of you in the periodic table.
Thus each proton and. The periodic table see figure below displays all of the known elements and is arranged in order of increasing atomic number. The electrons are arranged in shells around the nucleus.
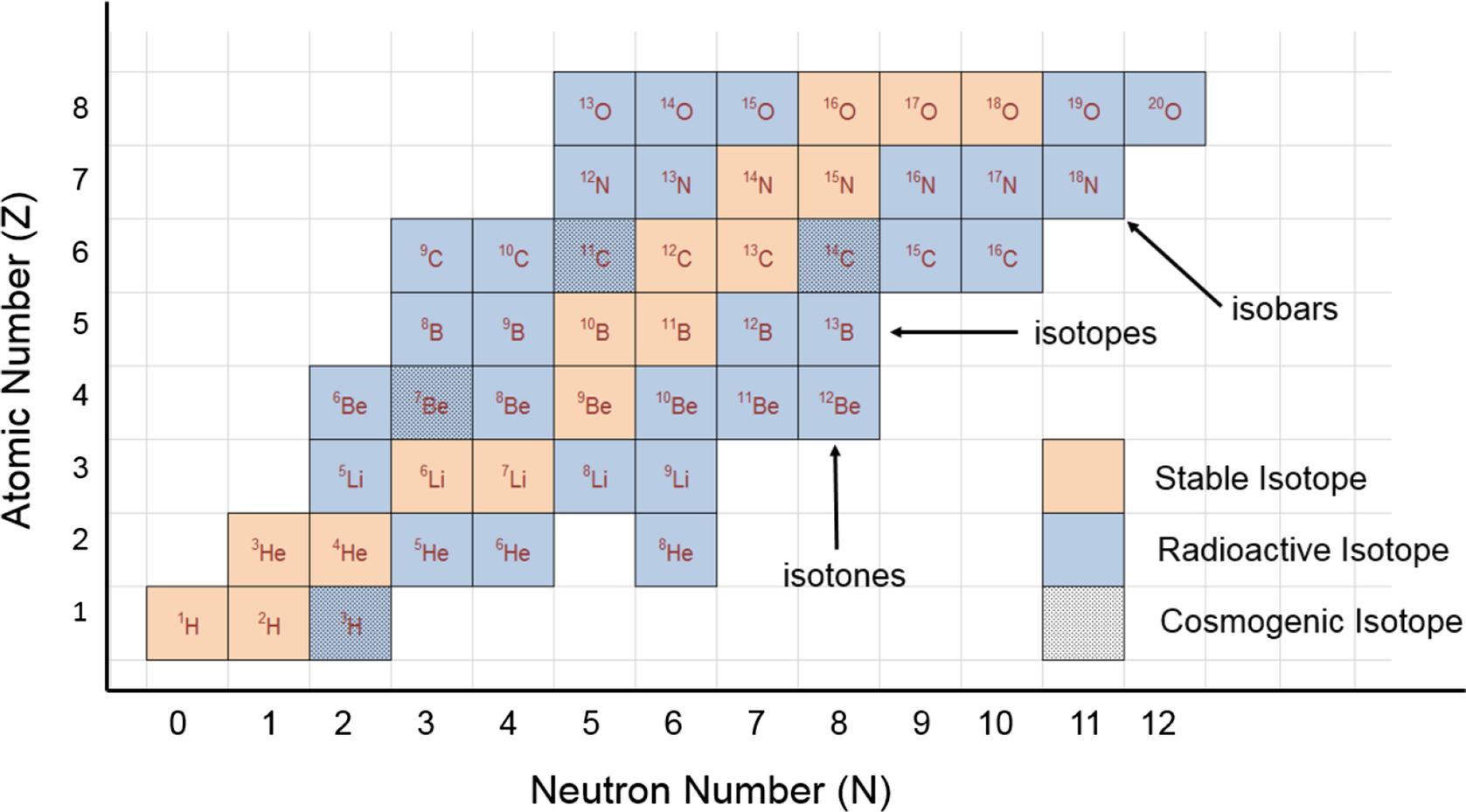
This isotope of carbon has 6 protons and 6 neutrons. Atomic mass number given for longest lived isotope. Atomic mass number given for longest lived isotope.
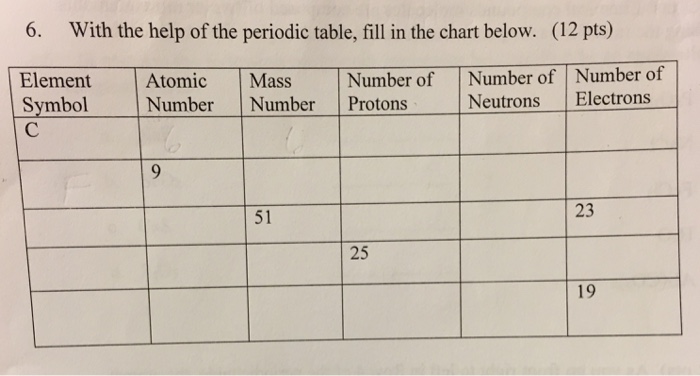
Atomic mass number given for longest lived isotope. The total number of protons and neutrons. Once you know where to look finding the number of protons neutrons and electrons will be a breeze.






















0 Response to "Periodic Table With Atomic Mass And Proton Number"
Post a Comment