Periodic Table Energy Levels And Sublevels
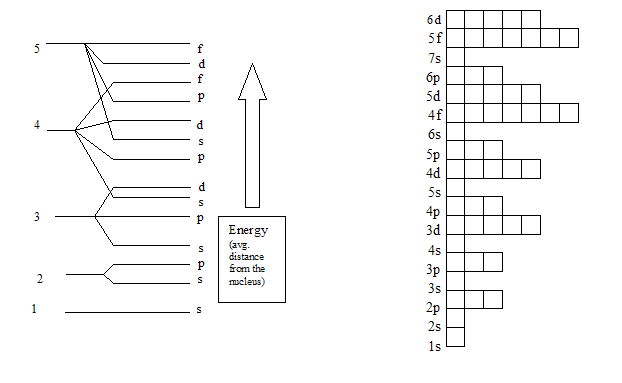
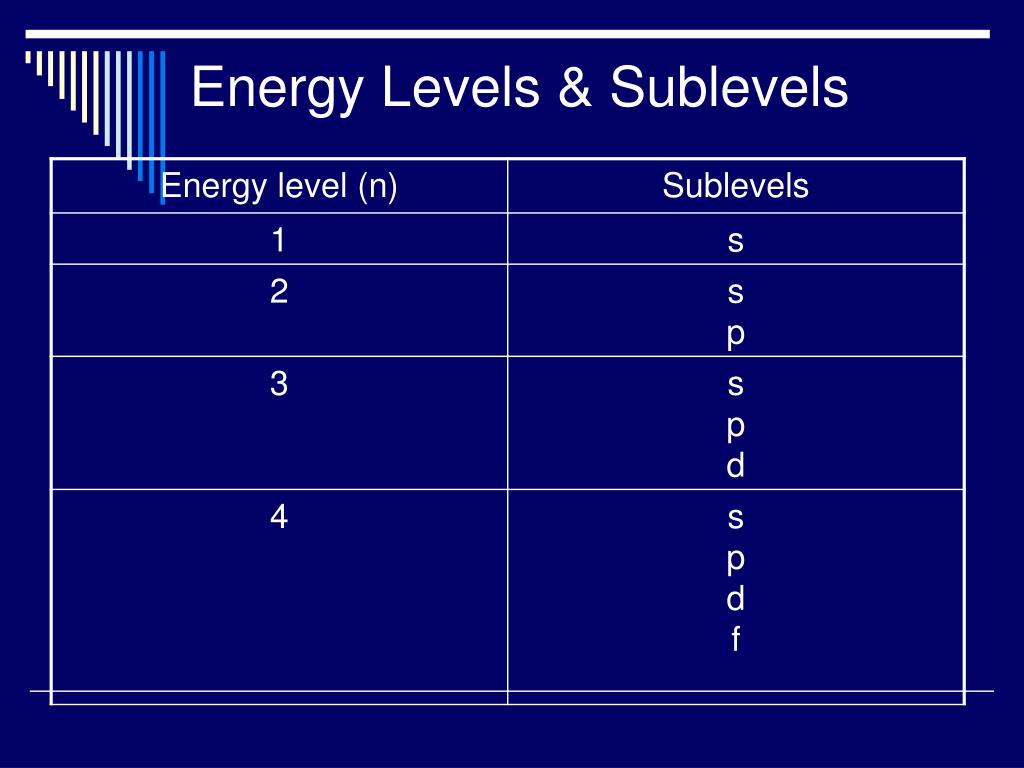
So for example electrons in the s sublevel of shell 3 have a different amount of energy from electrons in the p and d levels of shell 3. Electrons and sublevels electron configurations and the periodic table writing electron configurations box and arrow configurations using pauli exclusion principle and hunds rule quantum numbers.
Valence electrons are the number.
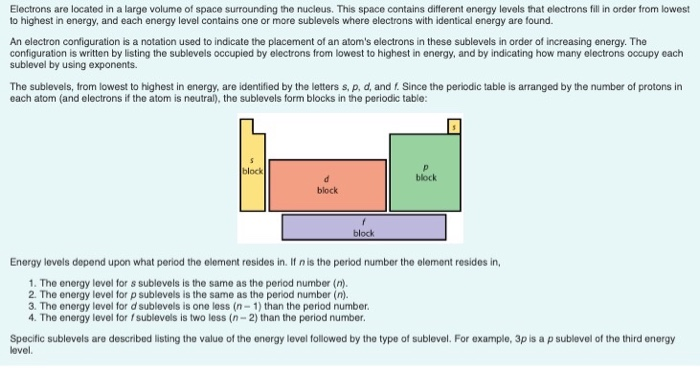
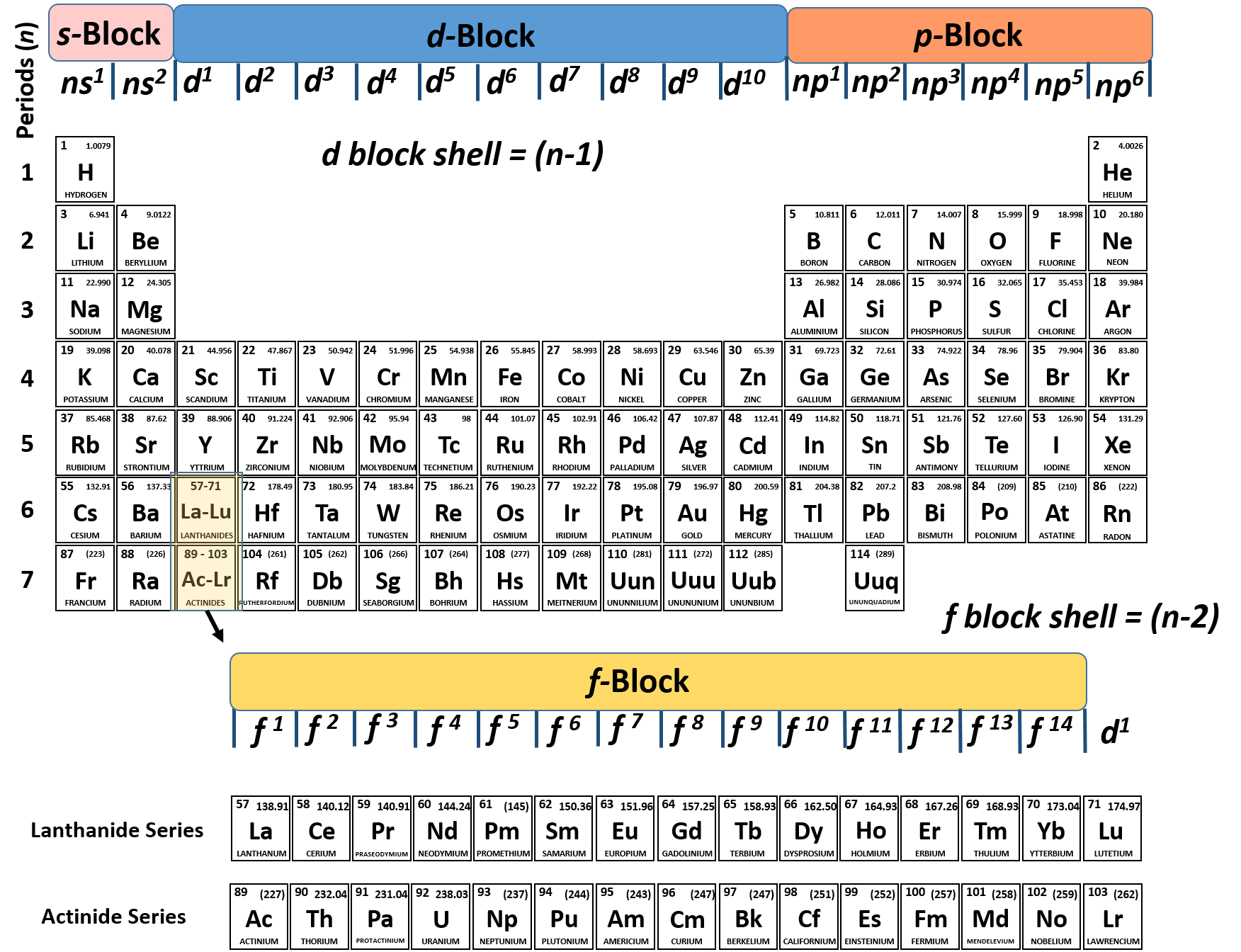
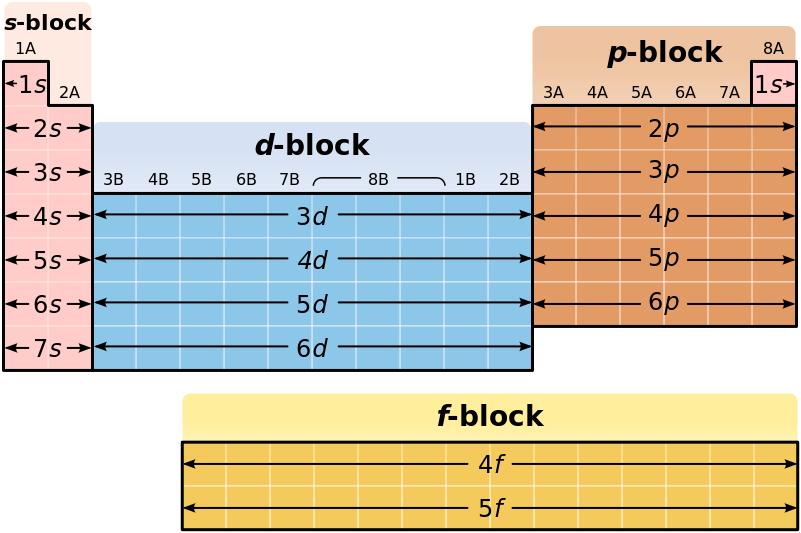
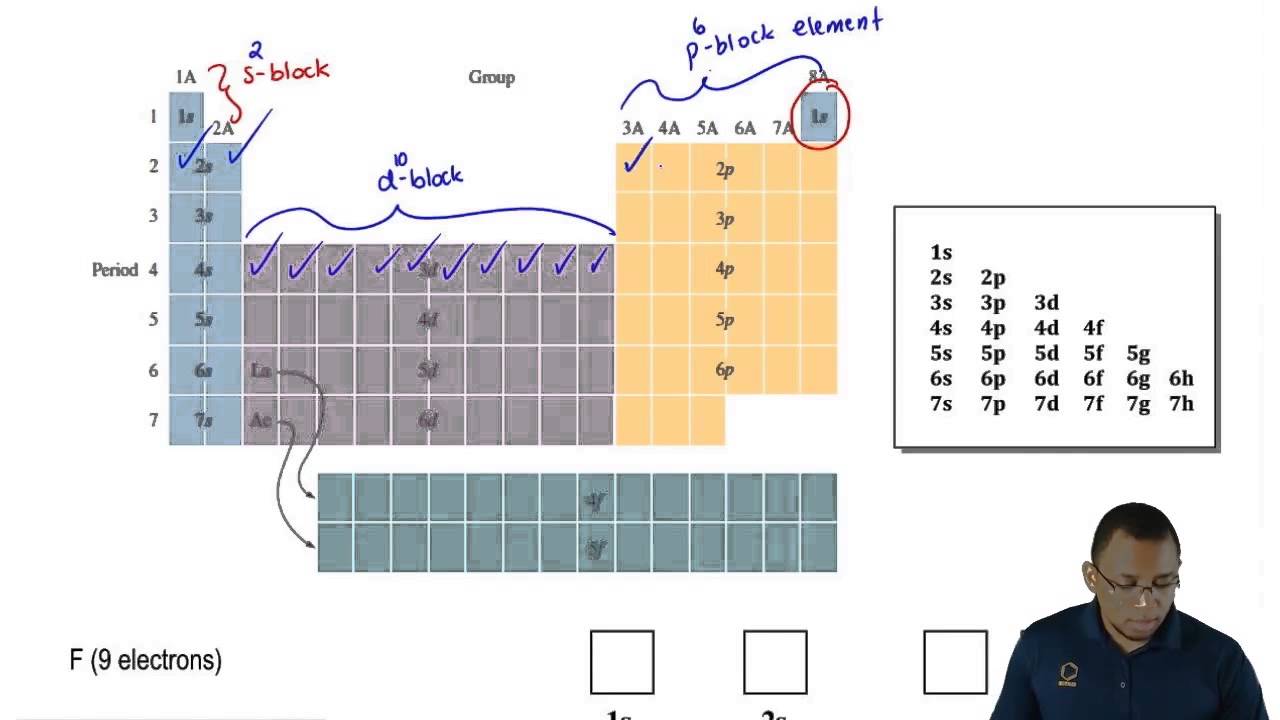
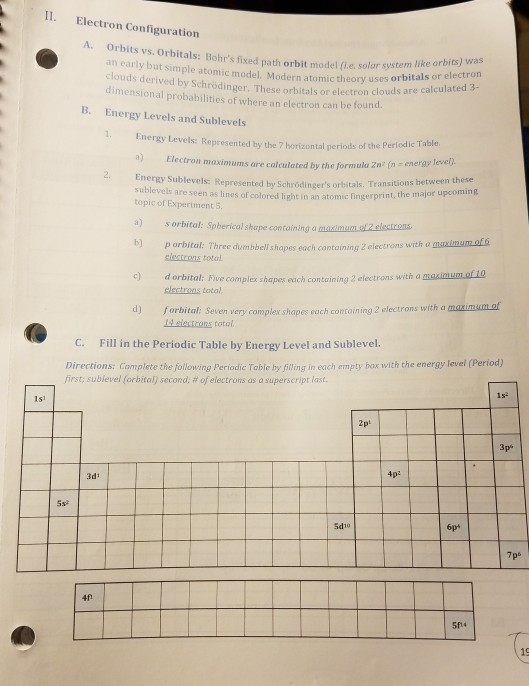
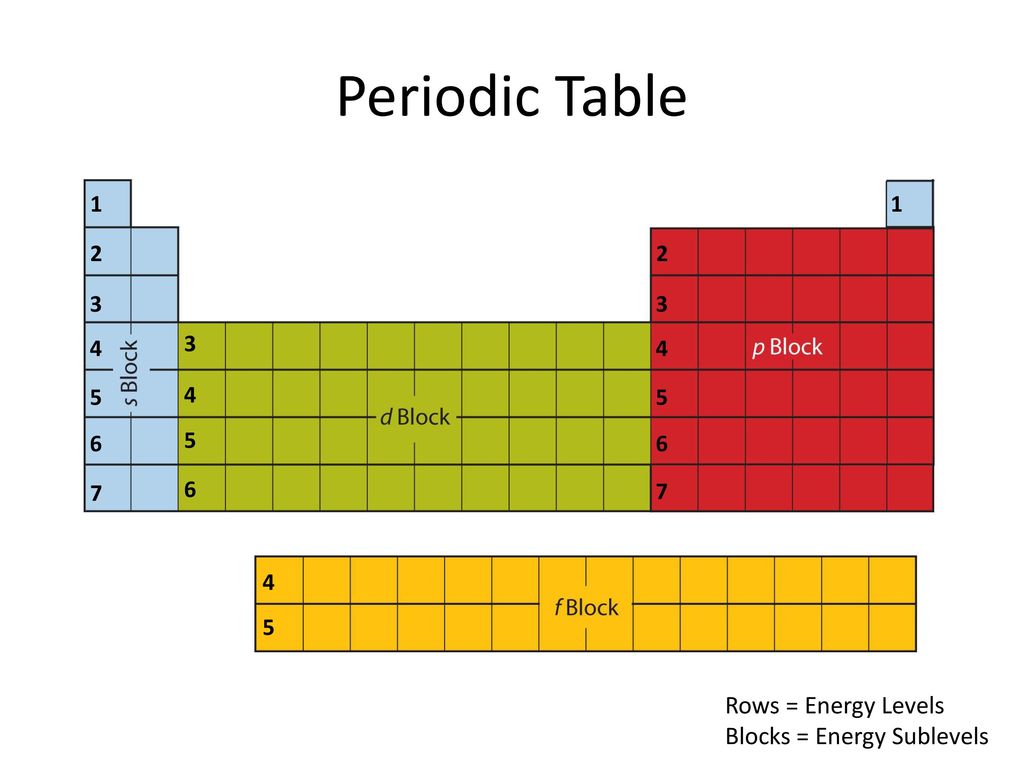
Periodic table energy levels and sublevels. Learn vocabulary terms and more with flashcards games and other study tools. The sublevels used with elements in energy level 6 period 6 are 6s 6p 4f and 5d orbitals. Energy levels energy sublevels orbitals pauli exclusion principle.
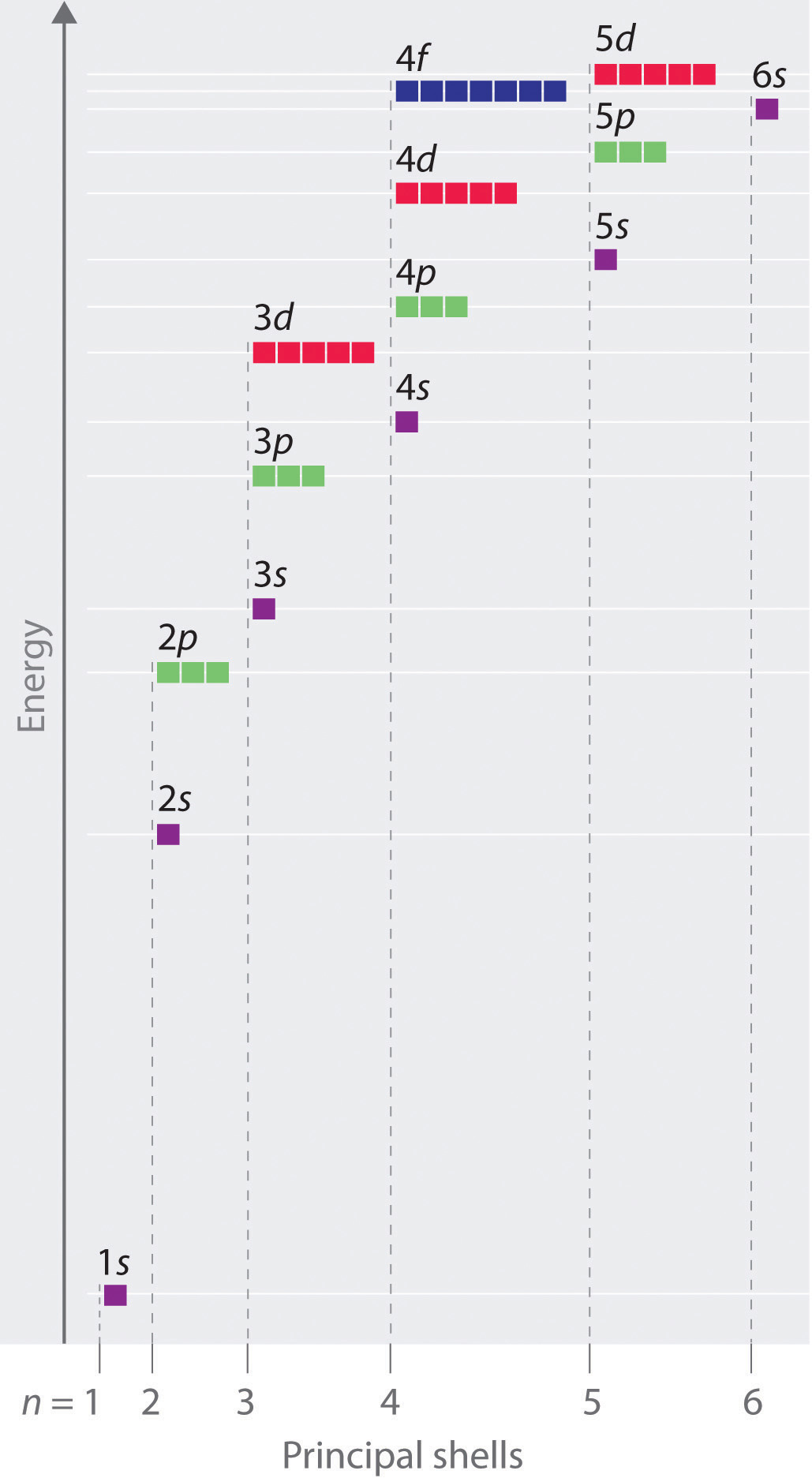
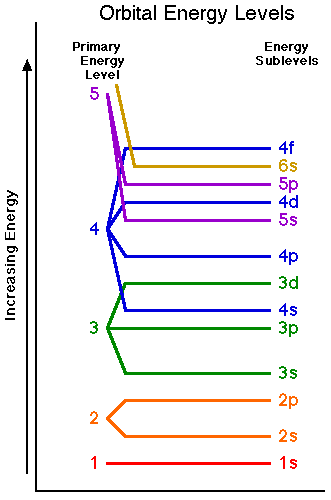
Httpbitly1yxzhvc subscribe for new. Visit our website for more of the help you need. The diagram below really shows the overlap of the principal energy levels.
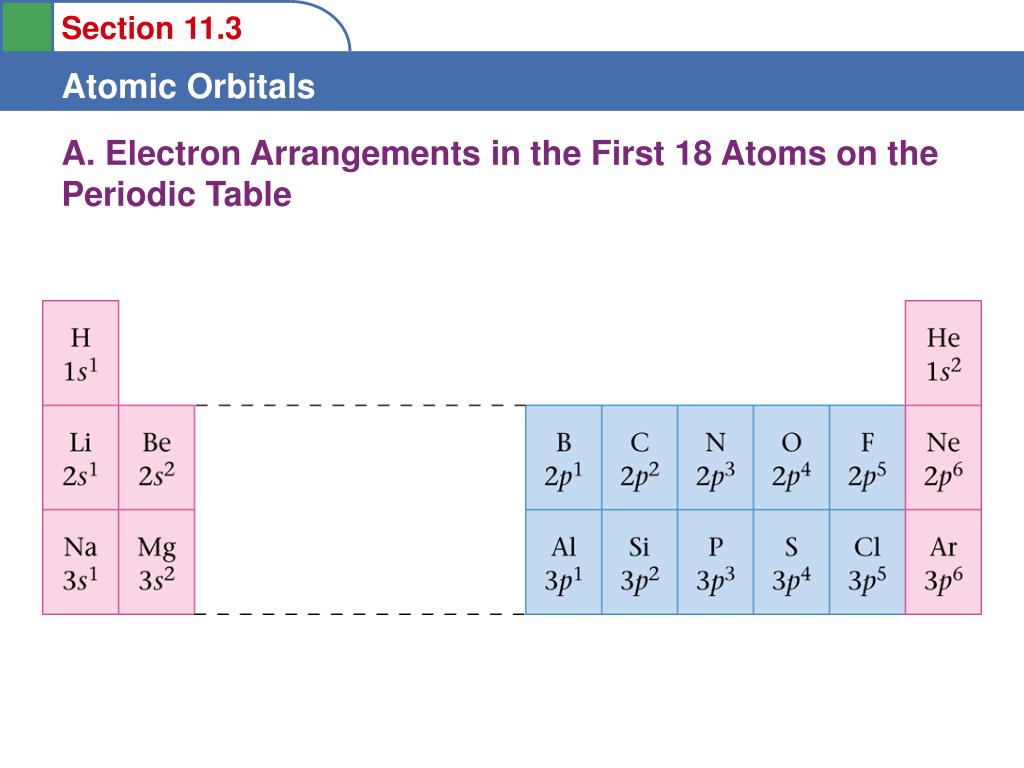
This video describes how the quantum mechanical model of the atom utilizes energy levels sublevels and orbitals to arrange electrons. Start studying atomic theory periodic table sublevels and orbitals. The elements in each column share similar properties and the same number of valence electrons.
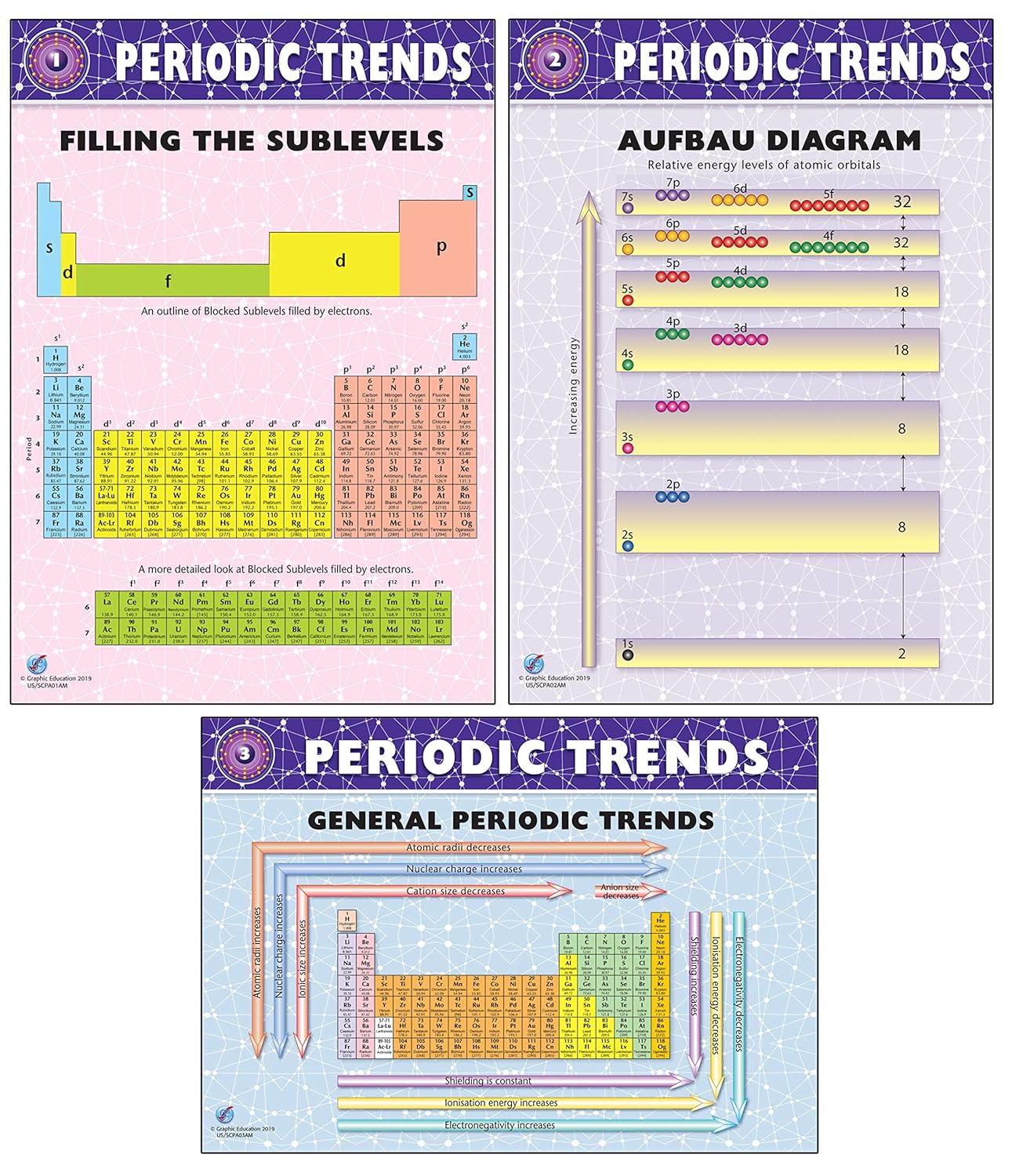
On to sublevels and the periodic table. The higher energy sub levels of some energy levels soon end up overlapping with the low energy sub levels of higher energy levels resulting in a more complex energy level diagram. Electron sublevels are known by the letters s p d and f.
Back to atomic structure links. Electron configurations are described in a separate video. All of hydrogens sublevels have the same energy because hydrogen only has one electron s p d and f sublevels.
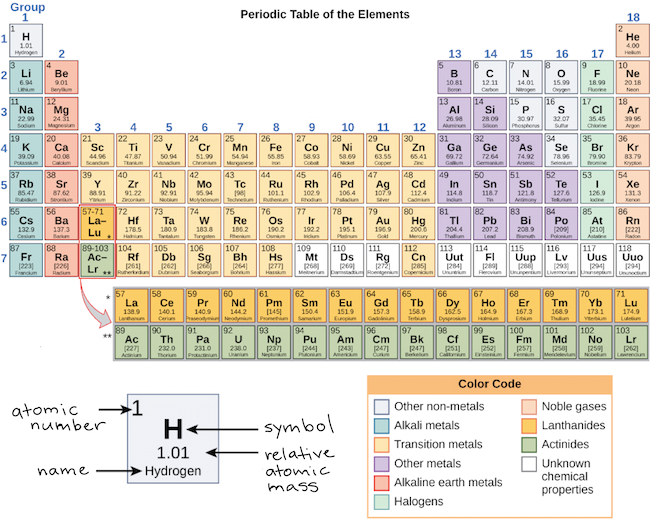
The periodic table is organized into columns and rows. Watch more of this topic at httpbitly1yxzcrf get more clutch. Each row represents an energy level.
The number of protons in the nucleus increases when reading the periodic table from right to left. Different sub levels have different energies and the energies of the different levels get closer and closer together with increasing energy level number. How many sublevels are used with elements on the periodic table in energy level 6.
This is not the case for hydrogen.



:max_bytes(150000):strip_icc()/periodic-table-of-the-elements-2017--illustration-769723031-5aa02f9b04d1cf00386ccf7c.jpg)



:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)







0 Response to "Periodic Table Energy Levels And Sublevels"
Post a Comment