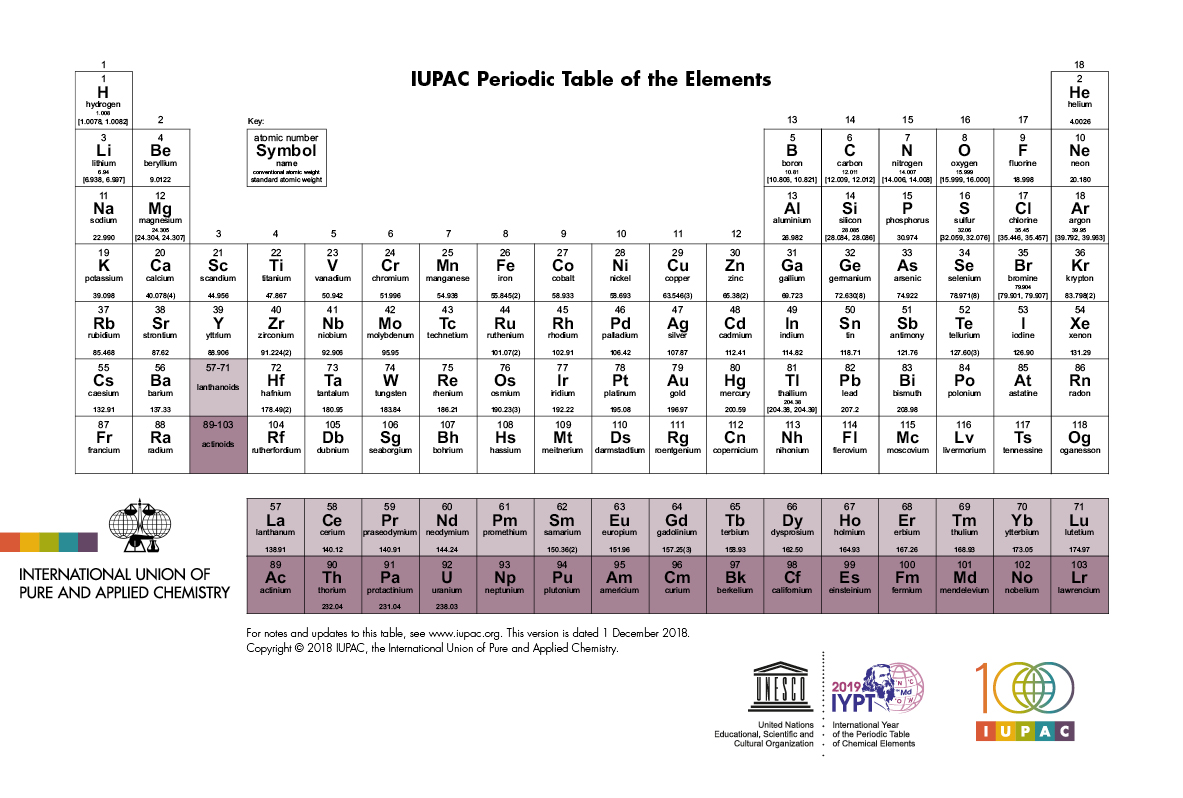
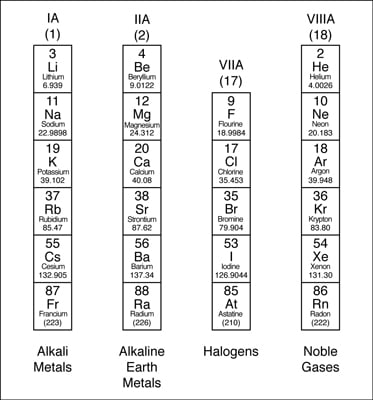
Periodic Table Definition
The seven rows of the periodic table are called periods. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search.
Periodic table definition is an arrangement of chemical elements based on the periodic law.
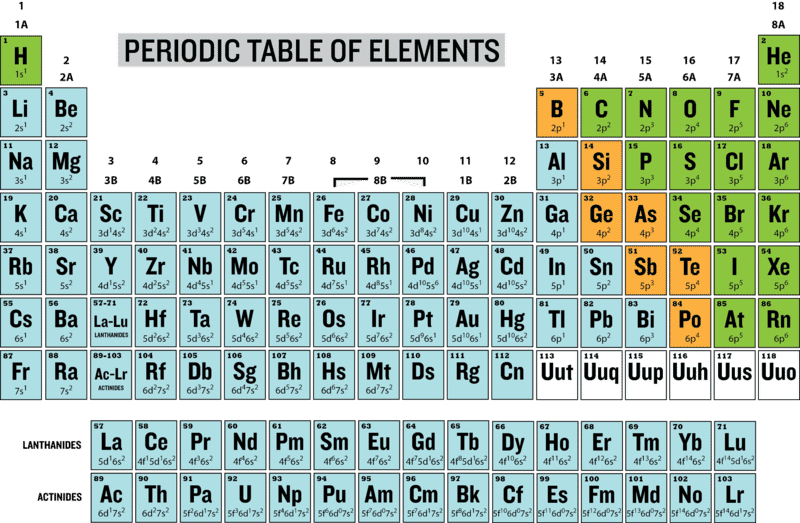
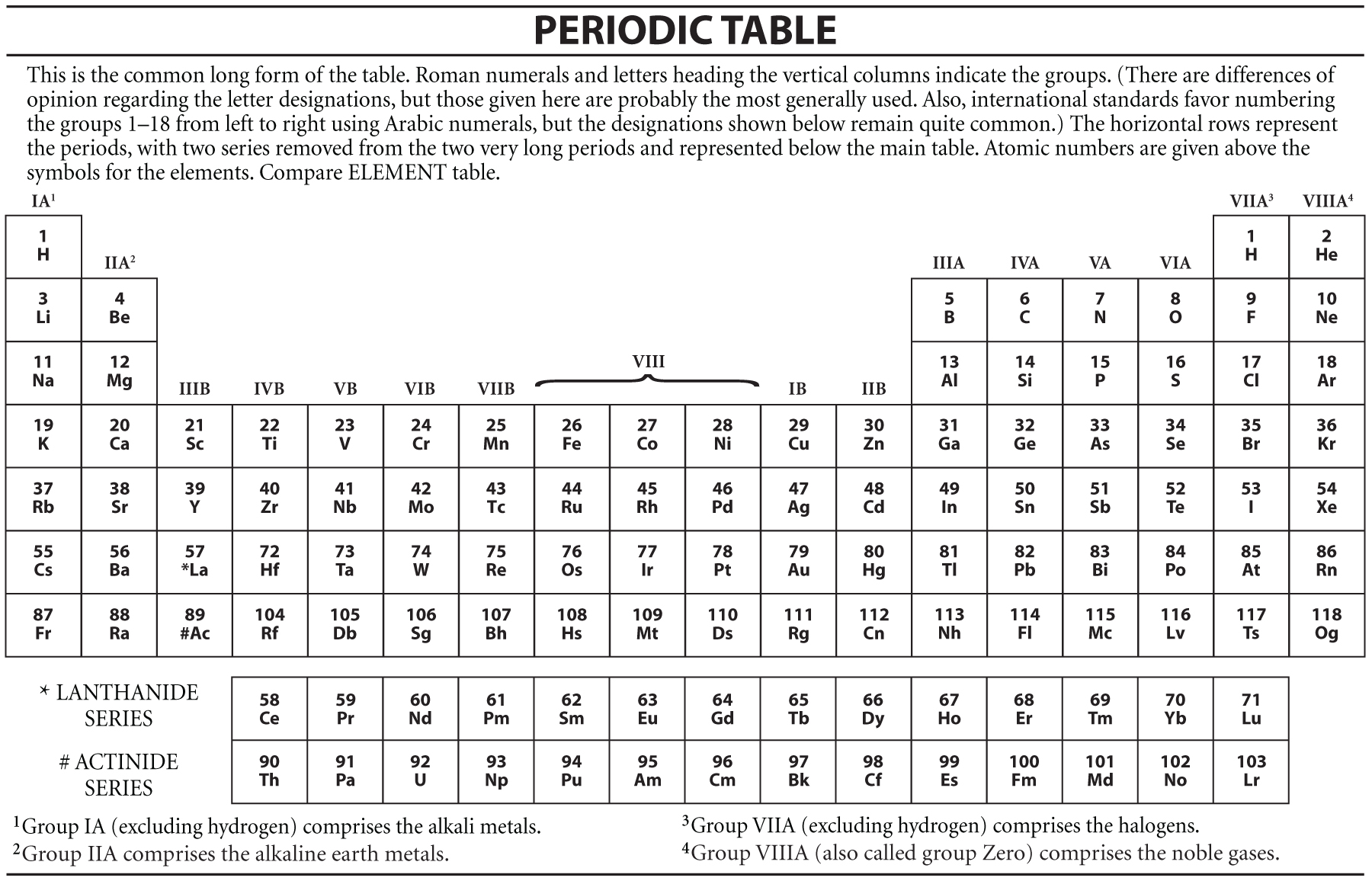
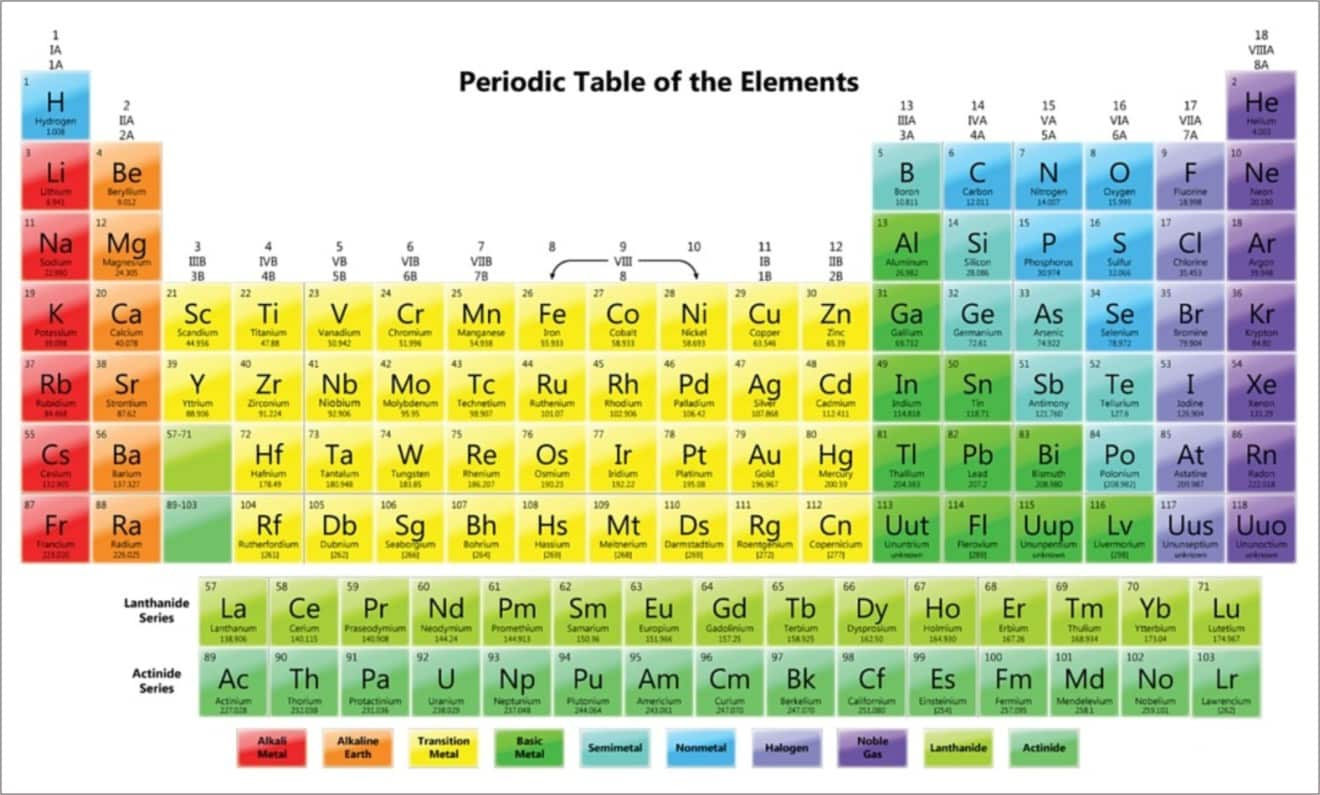
Periodic table definition. The periodic table has two rows at the bottom that are usually split out from the main body of the table. It is organized in order of increasing atomic number. The periodic table is a tabular arrangement of chemical elements that is arranged by increasing atomic number and groups elements according to recurring properties.
Chemistry the tabular arrangement of the elements according to their atomic numbers so that elements with similar properties are in the same column. Periodic table perdk n chemistry a table of the elements arranged in order of increasing atomic number based on the periodic law. Interactive periodic table of elements your complete guide to the elements including definition mass names of each chemical in the periodic table.
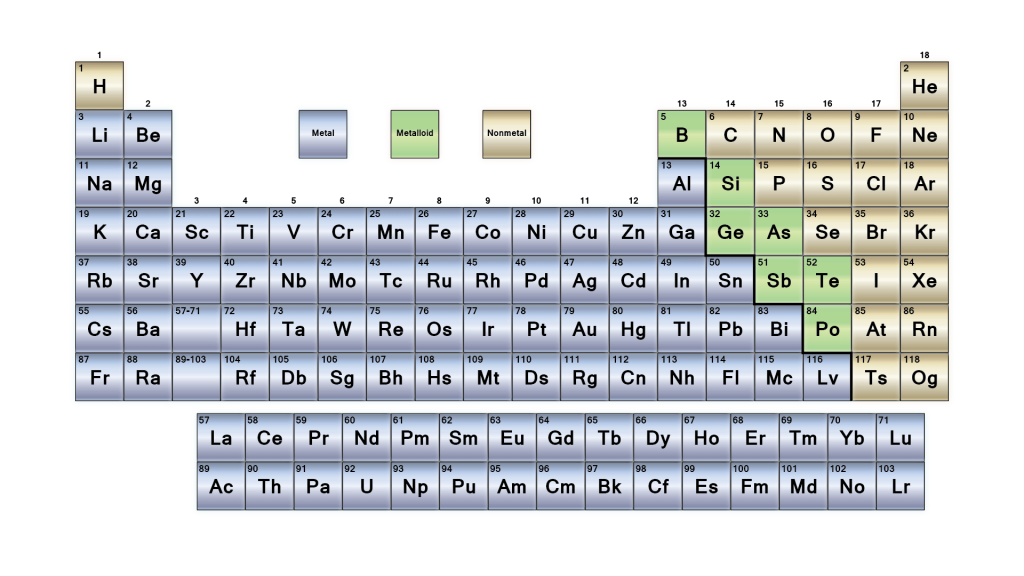
The periodic table is a tabular arrangement of the chemical elements. The rows are arranged so that metals are on the left side of the table and nonmetals are on the right side. Later 1902 mendeleev accepted the evidence for their existence and they could be placed in a new group 0 consistently and without breaking the periodic table principle.
The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical properties. Elements having similar. There is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties.
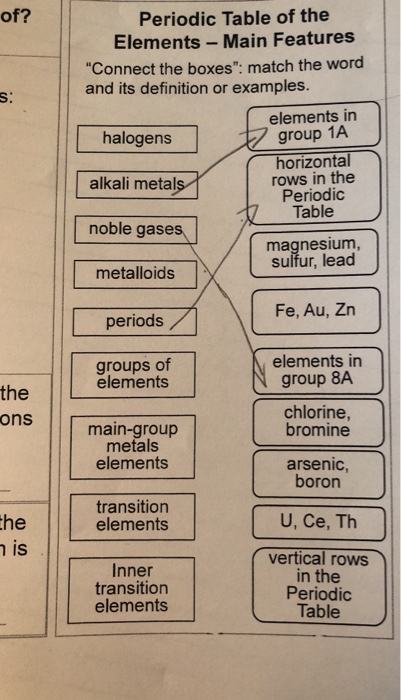
Full descriptions from write up sources. Periodic table periodic table n. R group name as recommended by iupac.
C group 18 the noble gases were not discovered at the time of mendeleevs original table. A table illustrating the periodic system in which the chemical elements formerly arranged in the order of their atomic weights and now according to their atomic numbers are shown in related groups. These rows contain elements in the lanthanoid and actinoid series usually from 57 to 71 lanthanum to lutetium and 89 to 103 actinium to lawrencium respectively.
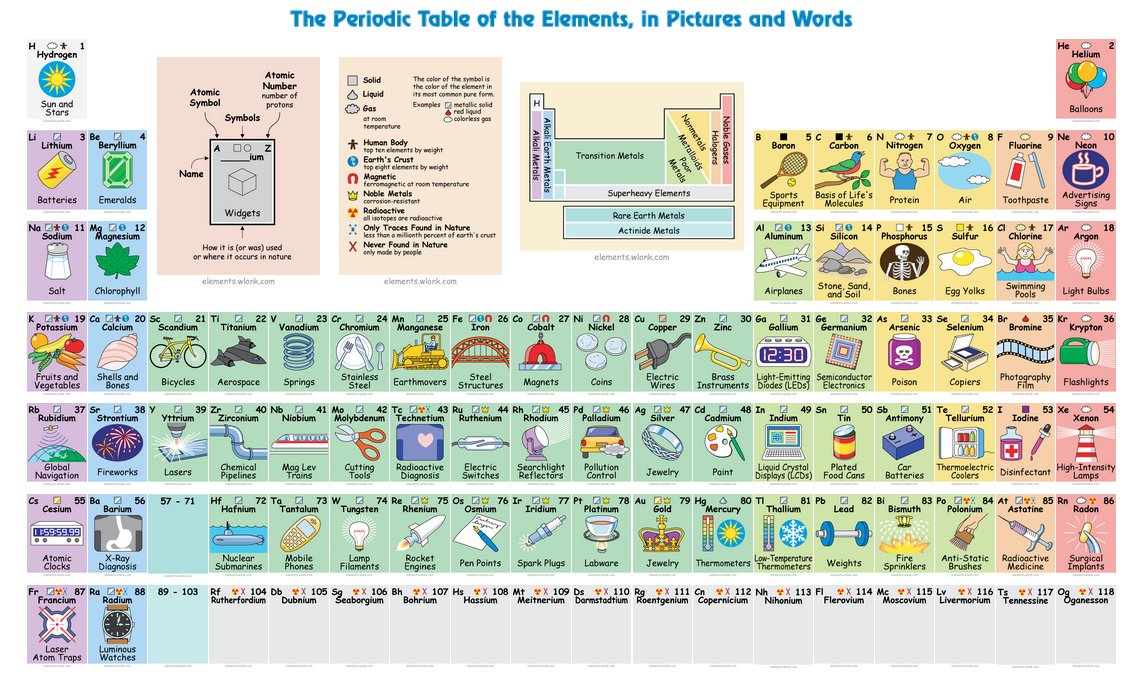









/periodic-table-165930186-590f2d703df78c92832fe141.jpg)











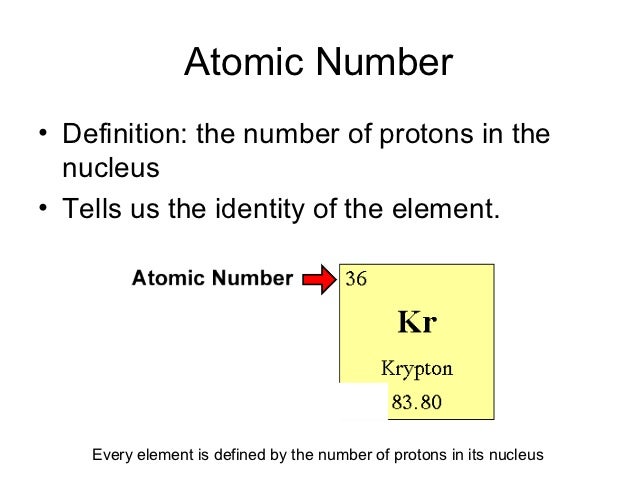

0 Response to "Periodic Table Definition"
Post a Comment