Periodic Table Cation And Anion Charges
Most other nonmetals typically form anions eg. Atoms that form these types of ions are called cations.
This table is also available as a free pdf downloadthere is also an older version of this table 2014 that you are welcome to use but the table below has slightly updated facts and is better optimized for printing.
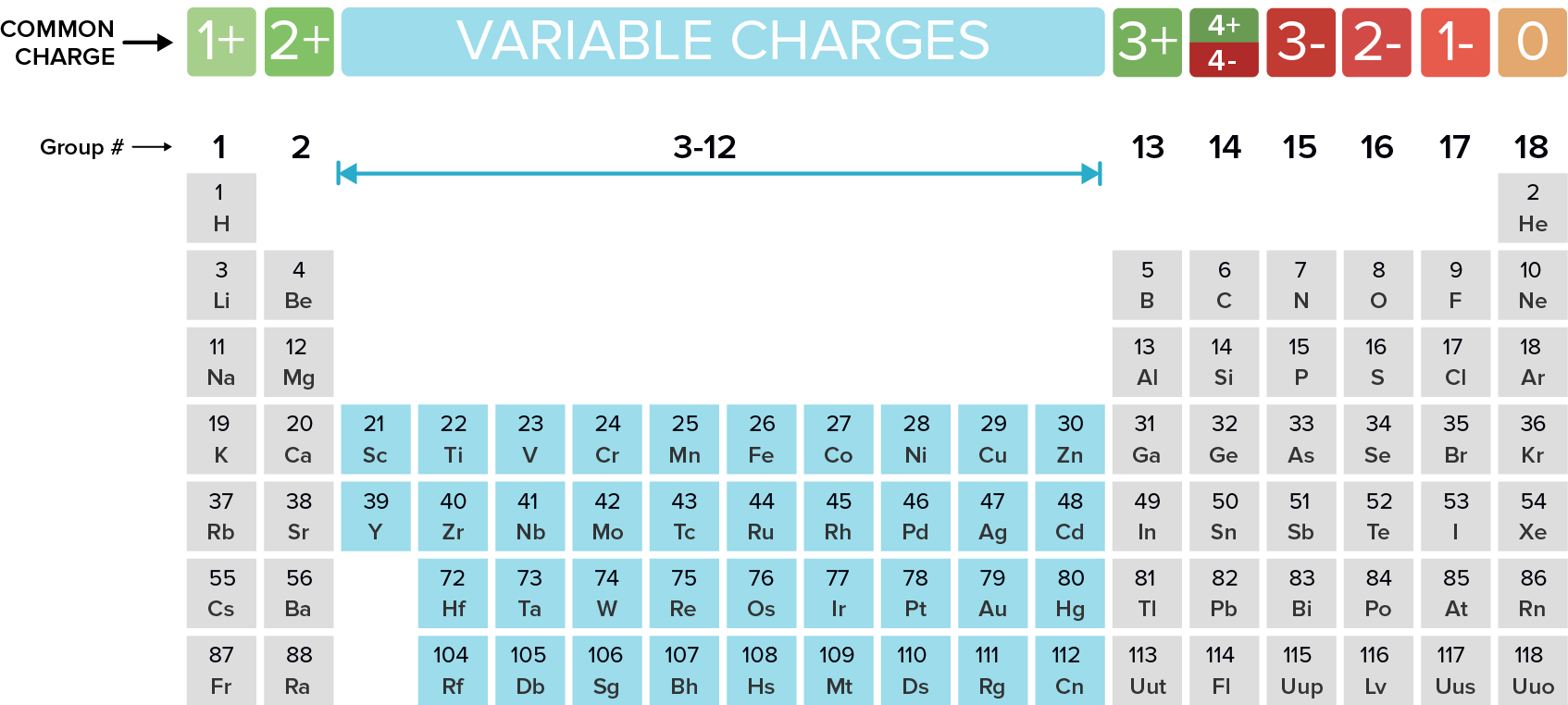
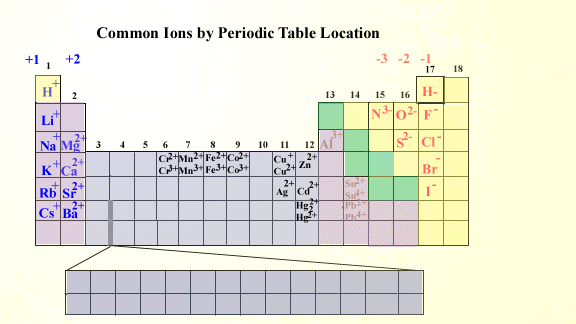
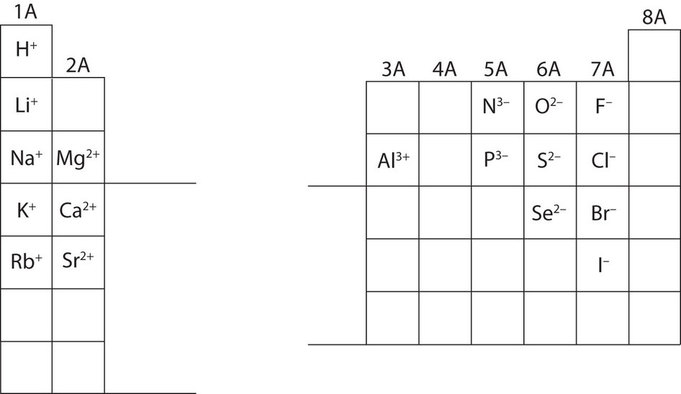
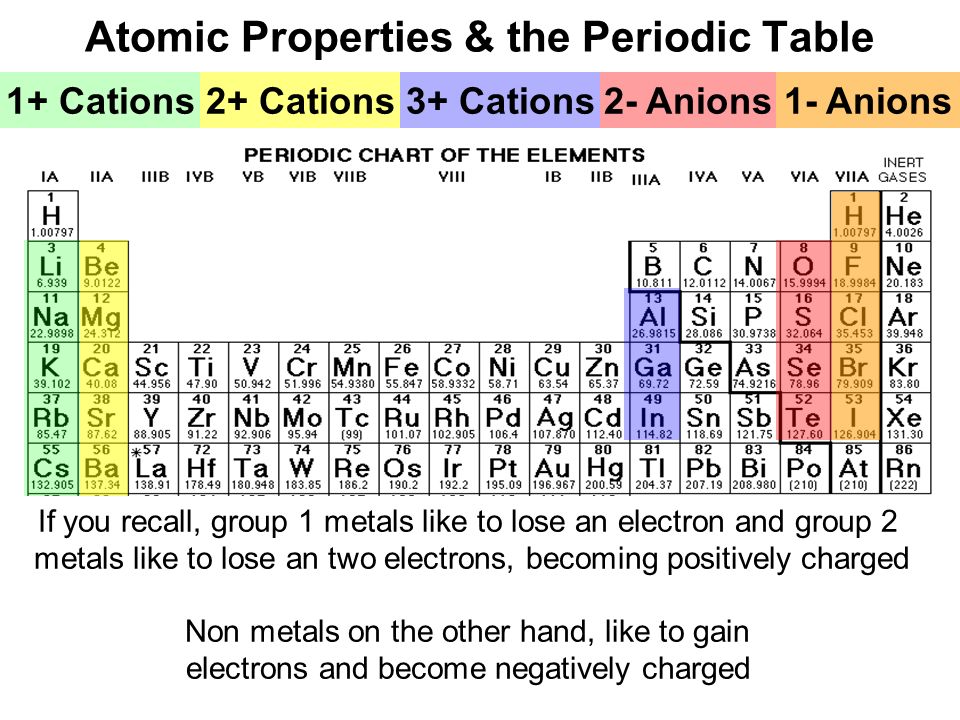
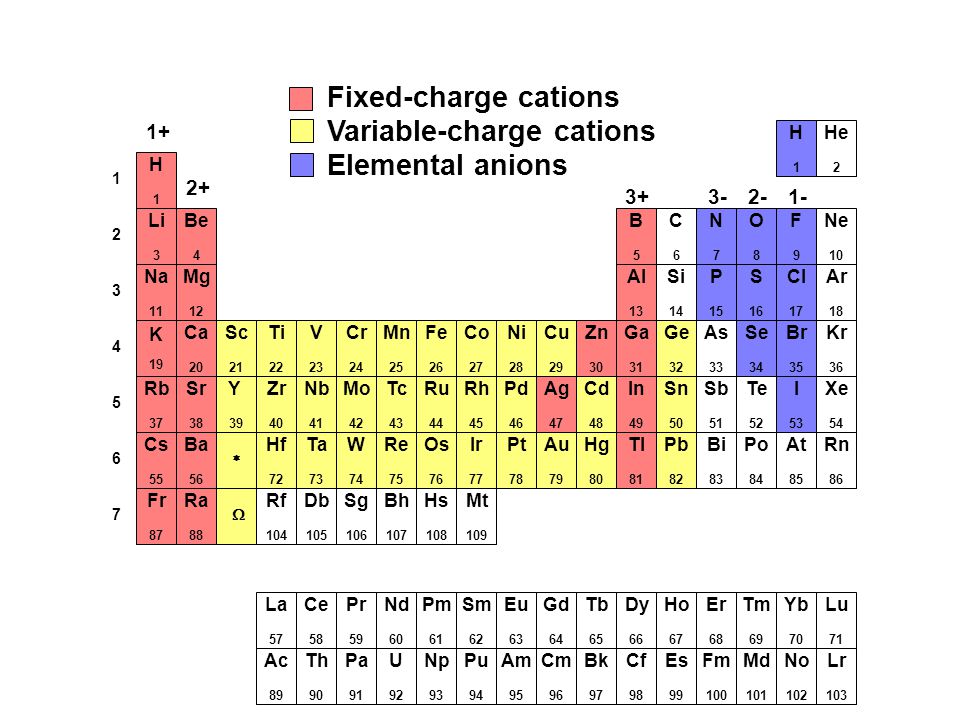
Periodic table cation and anion charges. Ions made from alkaline earth metals the second group on the periodic table have a 2 charge. The first table hows the family element and ion name for some common monoatomic one atom cations. Alkali metals and alkaline earth metals always form cations.
Metals generally form positively charged cations with a 2 charge. The va elements gain three electrons to form anions with a 3 charge. If you prefer a basic black and white table here you go.
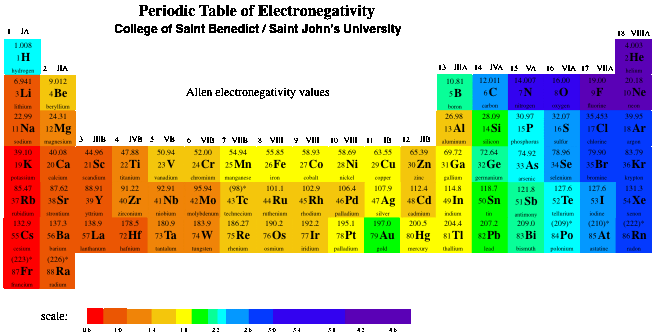
Oxygen nitrogen sulfur while most metals form cations eg. Sometimes you can predict whether an atom will form a cation or an anion based on its position on the periodic table. Wallpaper deep information for periodic table of elements charges likewise cation and anion periodic s image.
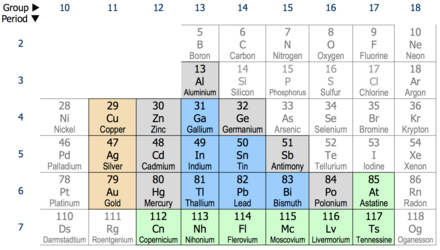
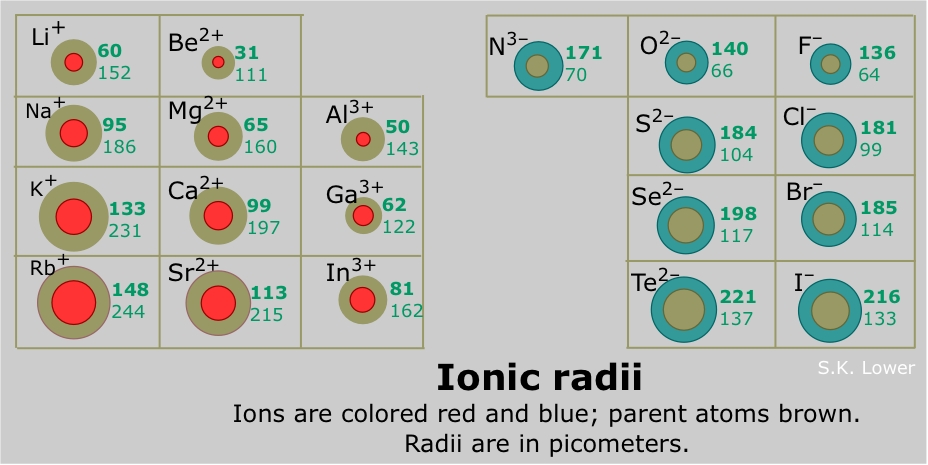
This wallpaper was ranked 37 by bing for keyword periodic table with charges you will find it result at bing. For example table salt or sodium chloride consists of the na cation bonded to the cl anion to form nacl. Figure pageindex3 shows how the charge on many ions can be predicted by the location of an element on the periodic table.
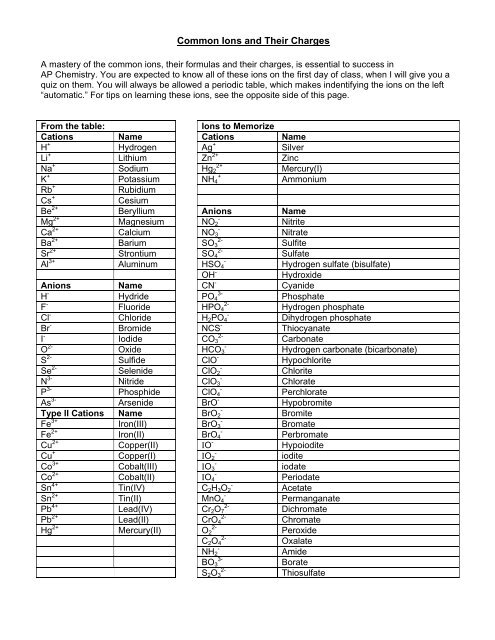
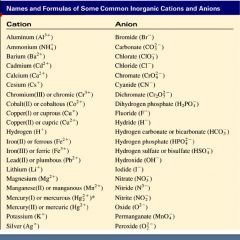
Salts are hygroscopic or tend to pick up waterthis water is called water of hydration. The main differences between cations and anions are summarized in the table below. An ion may consist of a single atom of an element a monatomic ion or monatomic cation or anion or of several atoms that are bonded together a polyatomic ion or polyatomic cation or anionbecause of their net electrical charge cations are repelled by other cations and are attracted to anions.
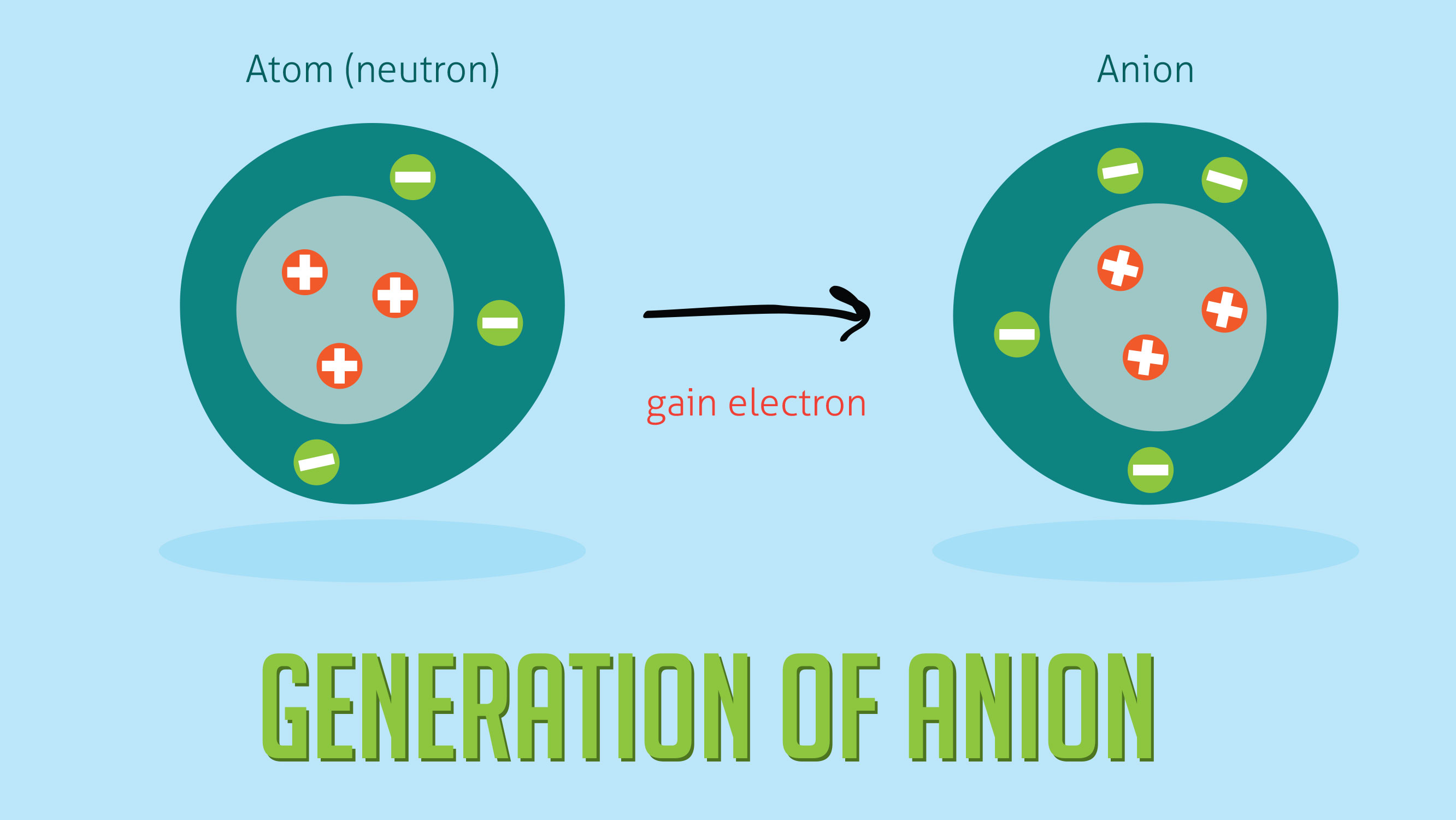
Black and white periodic table with charges. The position and groupings of elements in the periodic table are helpful when identifying the atoms that are most likely to form ions as well as whether or not those ions will be cations or anions. Neutral atoms become ions by either losing an electron to form positively charges cations or gaining electrons to form negatively charged anions.
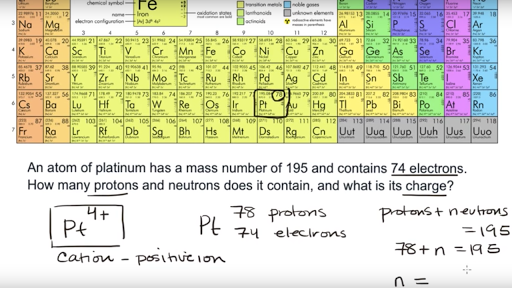
On the other side of the periodic table the next to last column the halogens form ions having a 1 charge. The resulting compound carries a neutral electrical charge. Cations are ions which have a positive electrical chargea cation has fewer electrons than protons.
We think its better but its up to you. Transition metals can form more than one kind of cation. Cation vs anion chart.
Metal atoms located on the left side of the periodic table always lose electrons to become cations. Salts are compounds composed of cations bonded to anions. The second table gives the same information for some common monoatomic anions.
Chlorine cl gains one electron to become cl whilst oxygen o gains two electrons to become o 2. Halogens form an anion. The number of electrons gained and so the charge of the ion is indicated after the chemical symbol eg.
Halogens always form anions. The via elements gain two electrons to form anions with a 2 charge.



















:max_bytes(150000):strip_icc()/cation-and-an-anion-differences-606111-v2_preview-5b44daf9c9e77c0037679d52.png)



0 Response to "Periodic Table Cation And Anion Charges"
Post a Comment