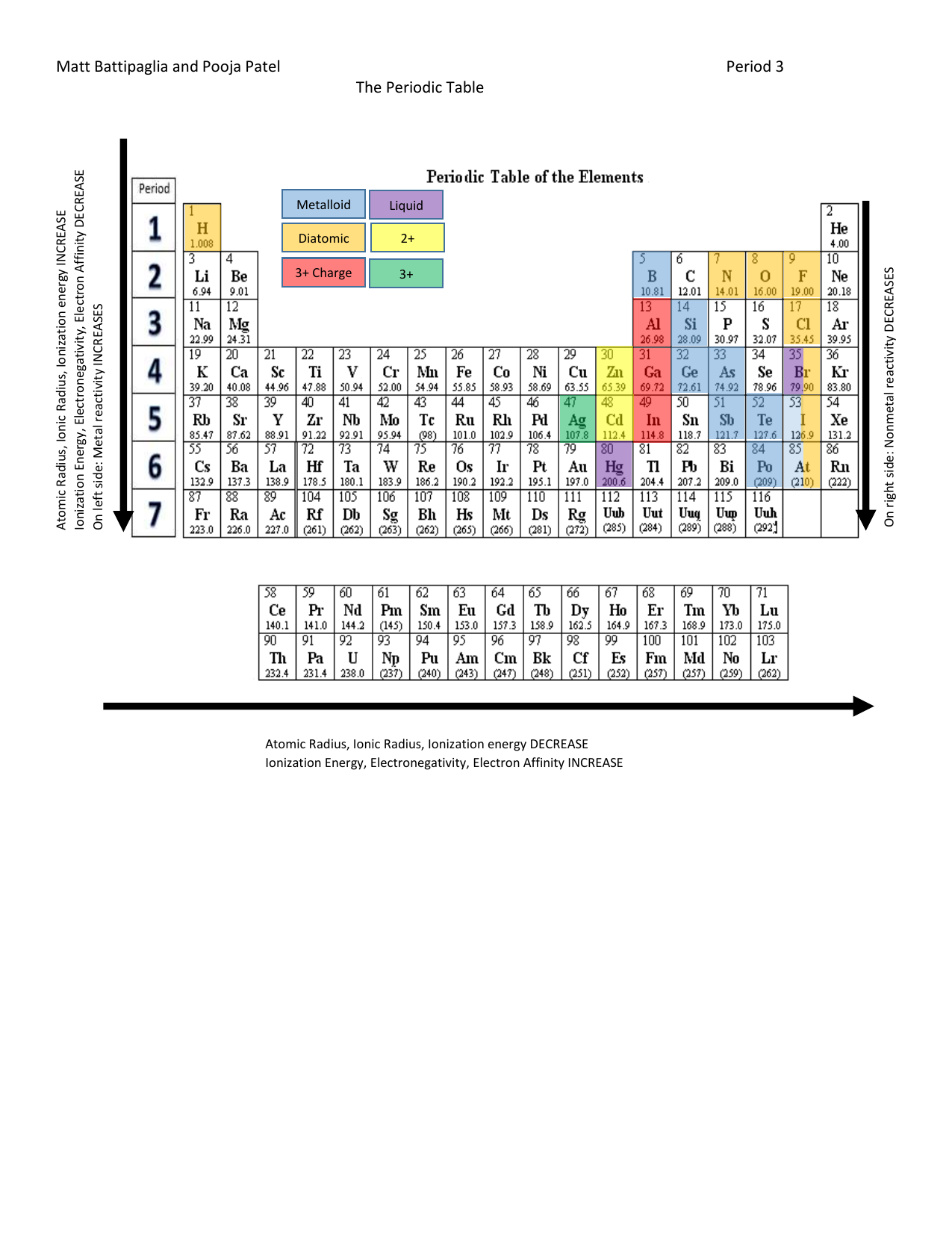
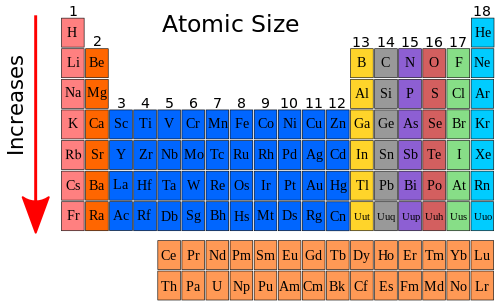
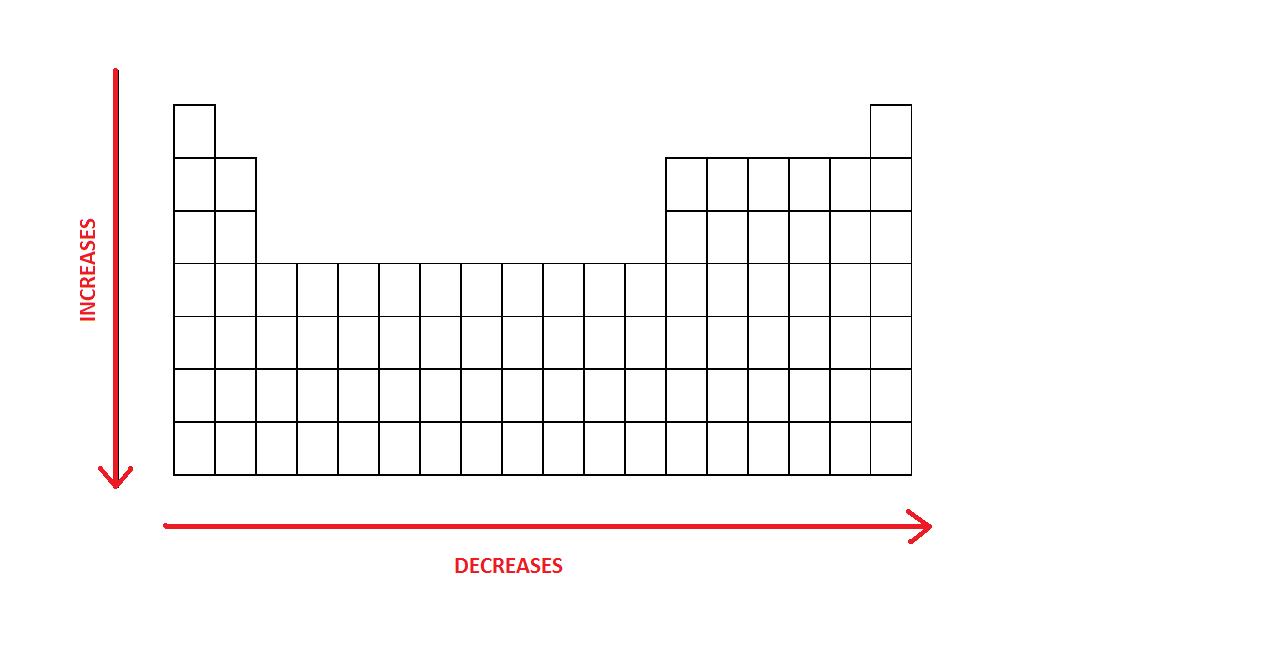
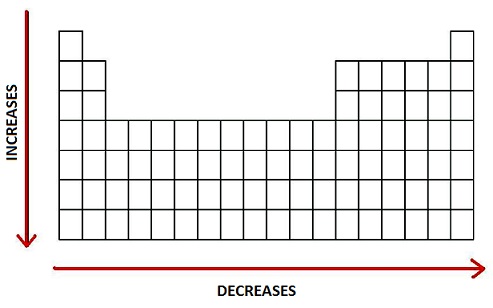
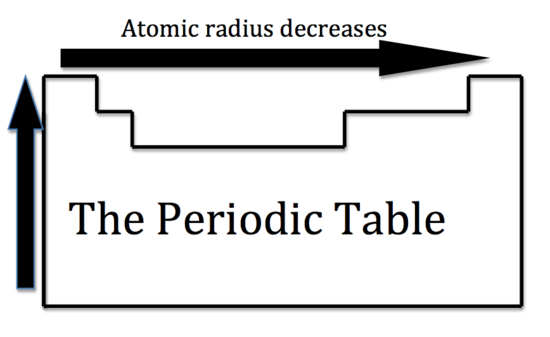
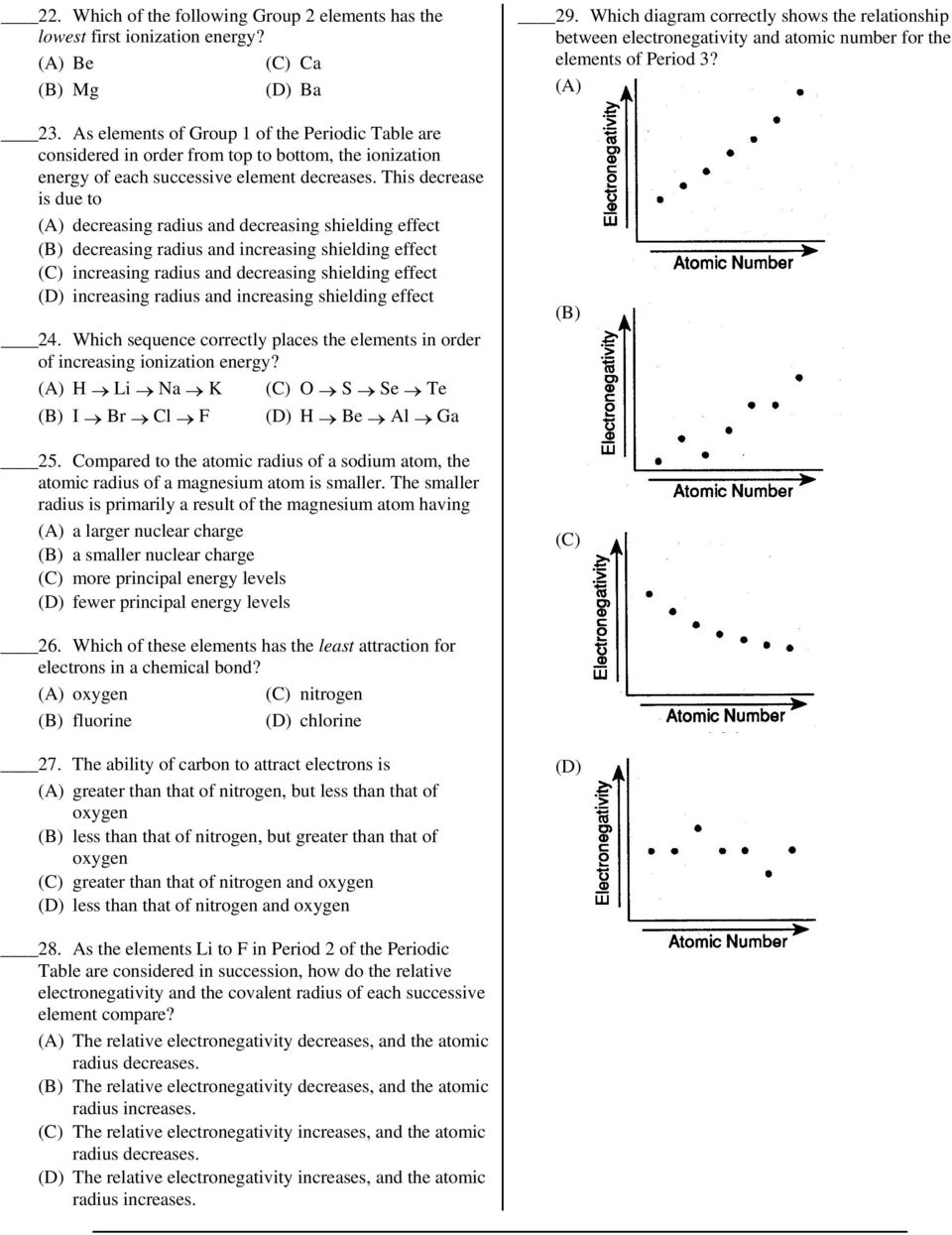
Decreasing Atomic Radius Periodic Table
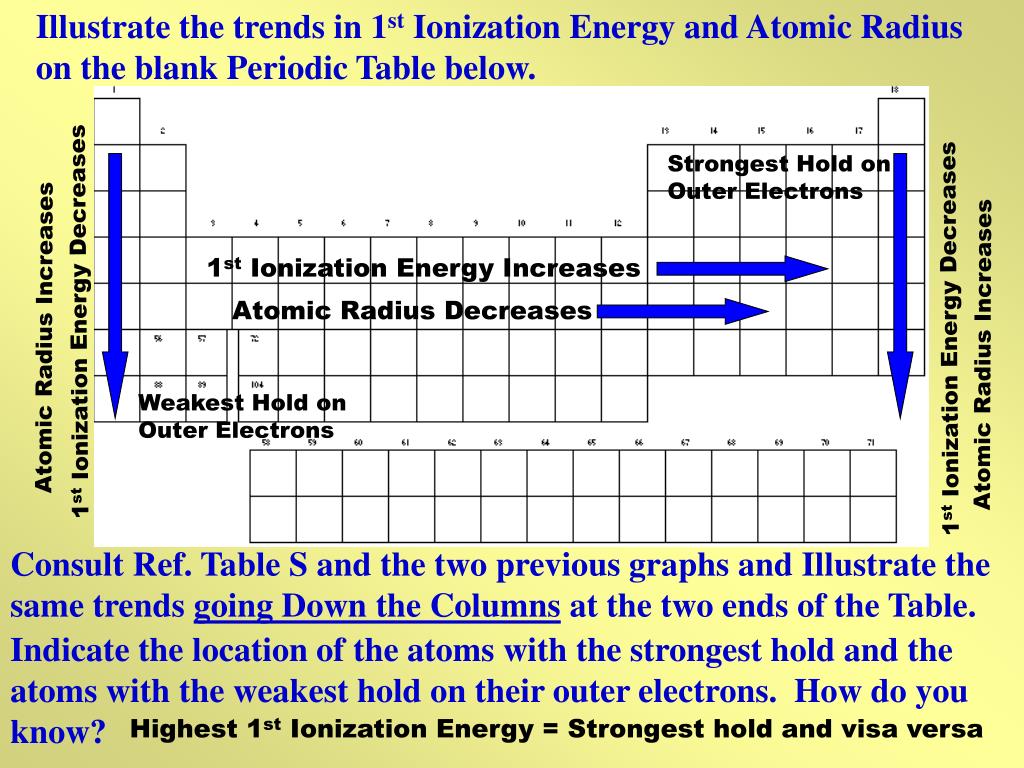
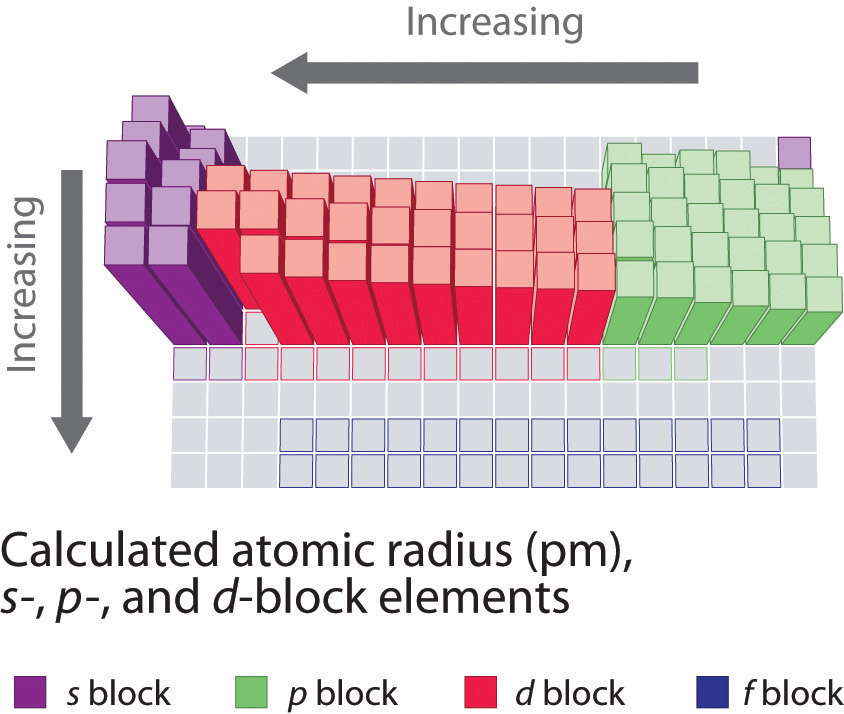
In a noble gas the outermost shell is completely filled. As you move down a group in the periodic table additional layers of electrons are being added which naturally causes the ionic radius to increase as you move down the periodic table.
So these are all different ways of thinking about it.
:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
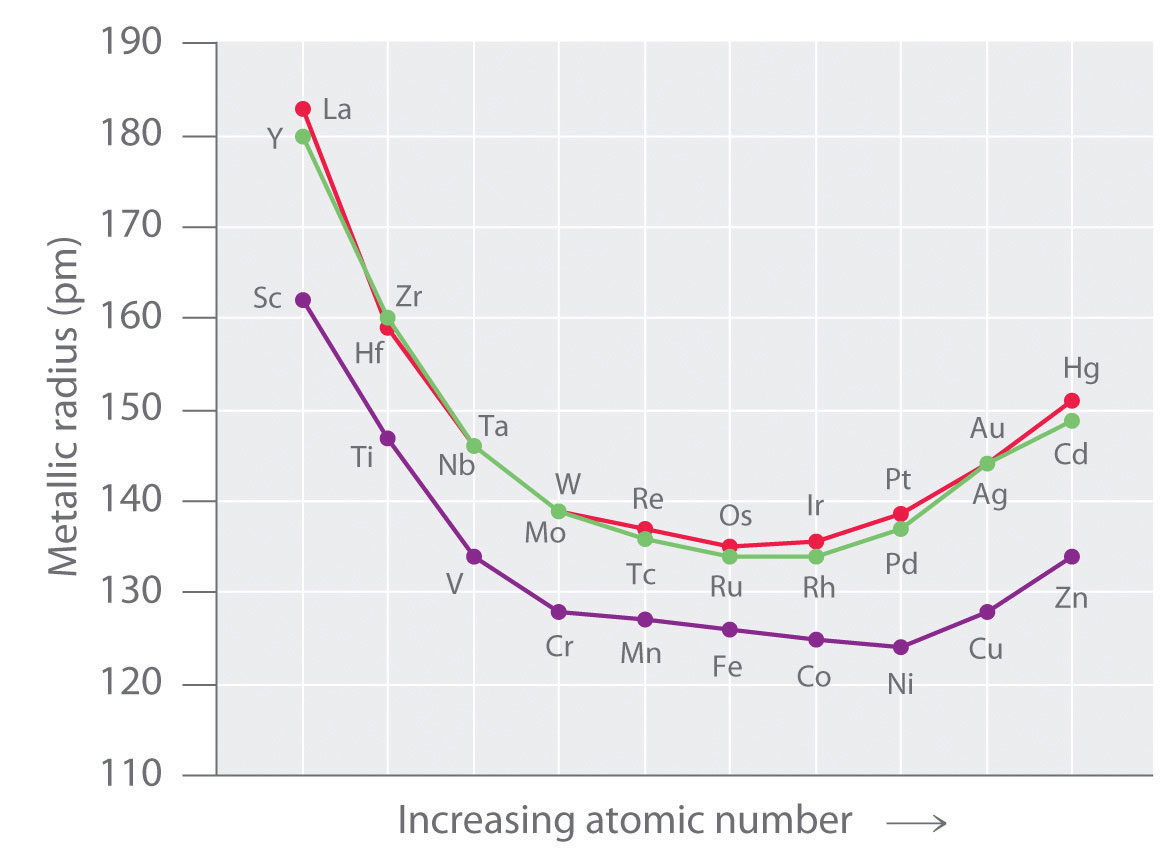
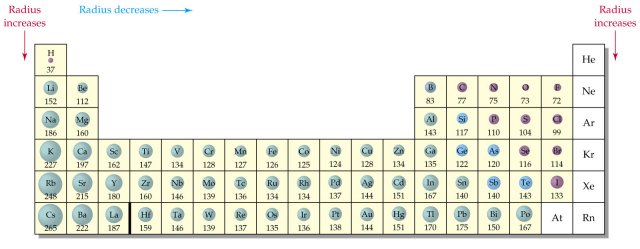
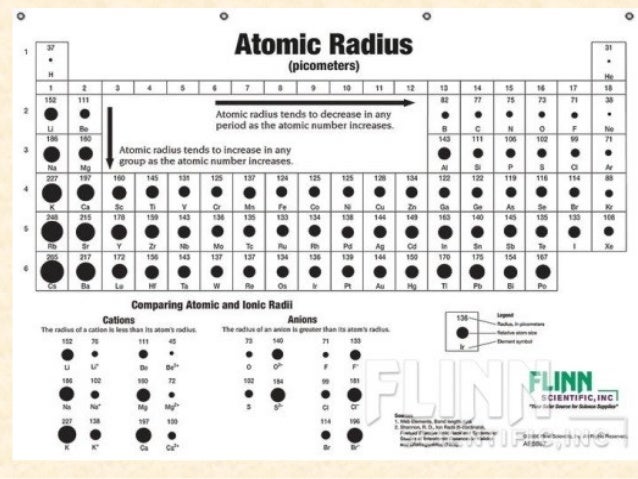
Decreasing atomic radius periodic table. The atomic radius of an element tends to increase the further down you go in an element group. The general trend of atomic radius is that it increases as you move down a group and decreases as you move to the right across a period. As the atomic number increases along each row of the periodic table the additional electrons go into the same outermost shell.
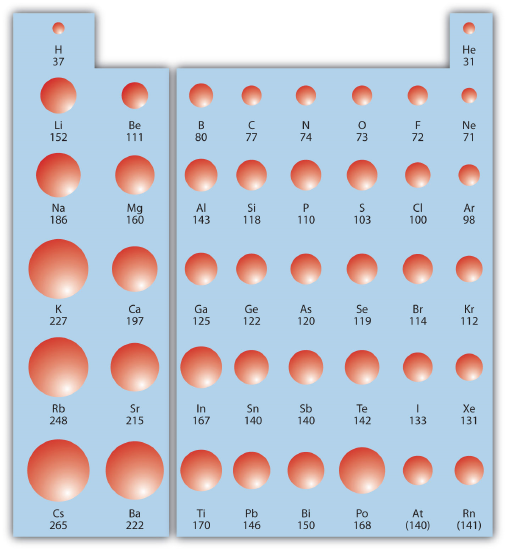
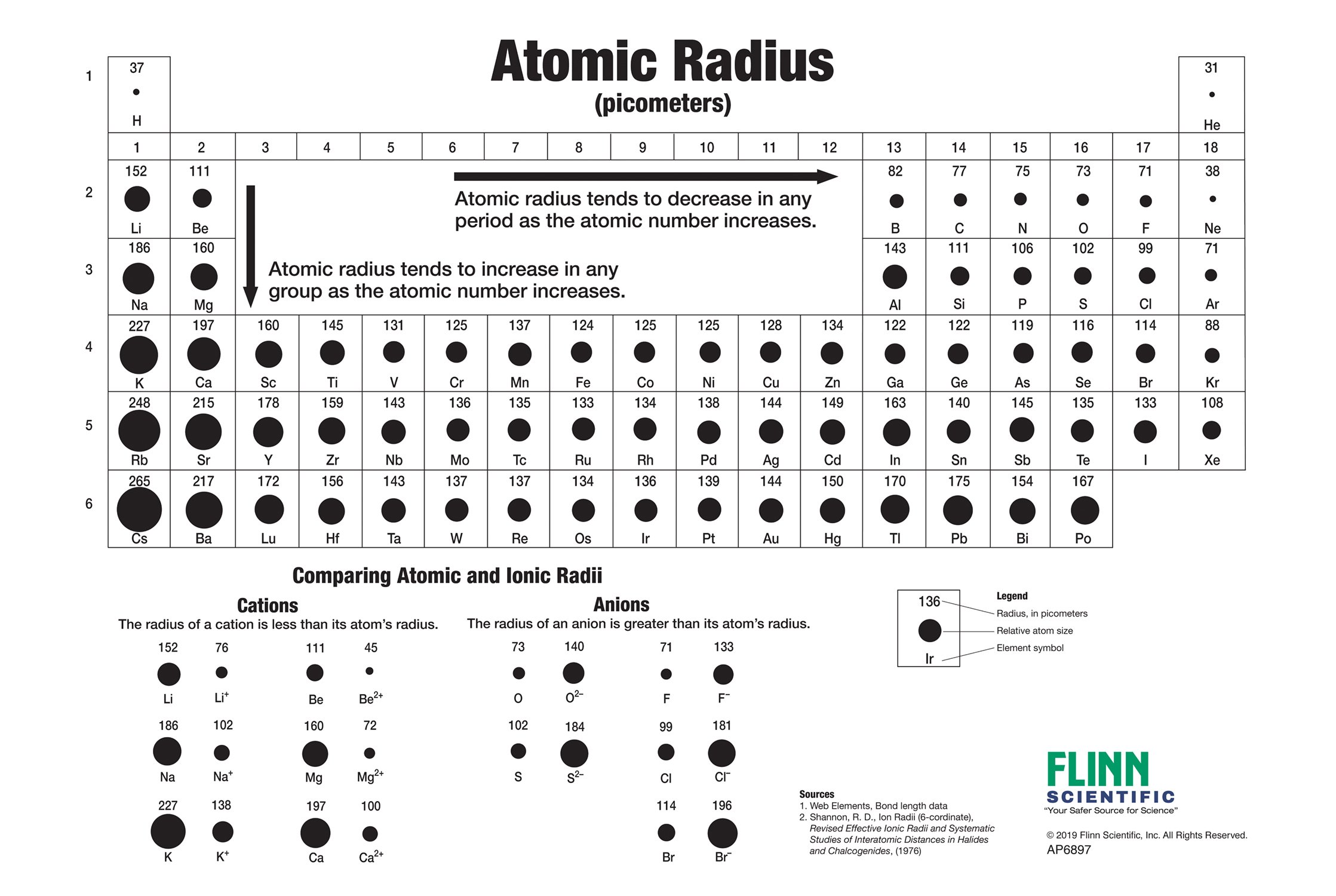
Periodic table of elements sorted by atomic radius. Click here to buy a book photographic periodic table poster card deck or 3d print based on the images you see here. In the periodic table elements are categorized based on their electronic structure.
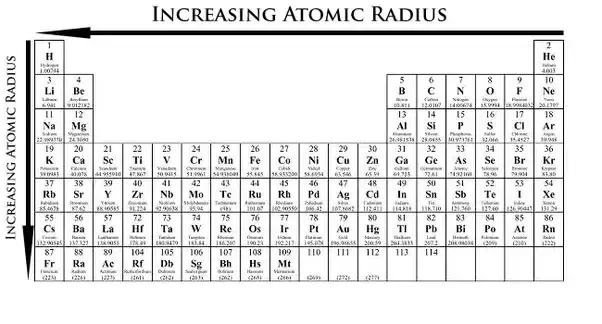
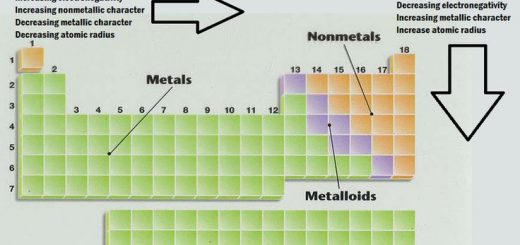
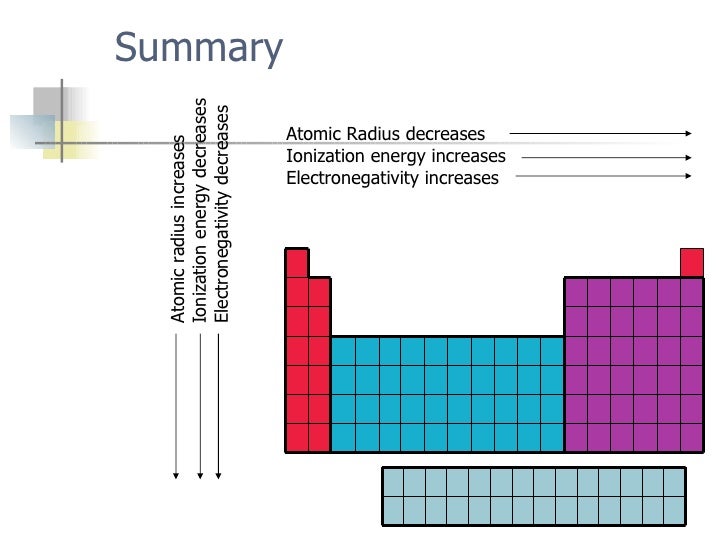
Now with that out of the way lets think about what the trends for atomic size or atomic radii would be in the periodic table. This makes the recurring element properties noticeable in this table. Here is a look at the periodic table trends of electronegativity atomic radius electron affinity metallic character and ionization energy.
Decreasing atomic radii periodic table. Core charge protons non valence electrons. If you need to cite this page you can copy this text.
2222007 citing this page. Whose radius gradually contracts due to the increasing nuclear charge. Atomic radius trend ik png figure 6 periodic table atomic radius trends in the periodic table periodictabledraft15 gif periodic table graphics atomic radii vfi.
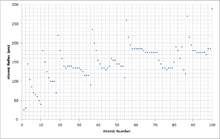
Why does radius increase with higher atomic numbers in a group. Atomic radius can be linked to core charge. Atomic radius periodic table trends no matter what criteria you use to describe the atomic radius the size of an atom is dependent on how far out its electrons extend.
And so when you have a covalent bond like this you can then find the distance between the 2 nuclei and take half of that and call that call that the atomic radius. The periodic table of the elements including atomic radius 1 18 hydrogen 1 h 101 31 2 alkali metals alkaline earth metals transition metals lanthanides actinides other metals metalloids semi metal atomic radius nonmetals 694 halogens noble gases element name 80 symbol beryllium picometers mercury hg 20059 132 atomic lithium avg. Therefore the additional electron of next alkali metal will go into the next outer shell accounting for the sudden increase in the atomic radius.





















0 Response to "Decreasing Atomic Radius Periodic Table"
Post a Comment