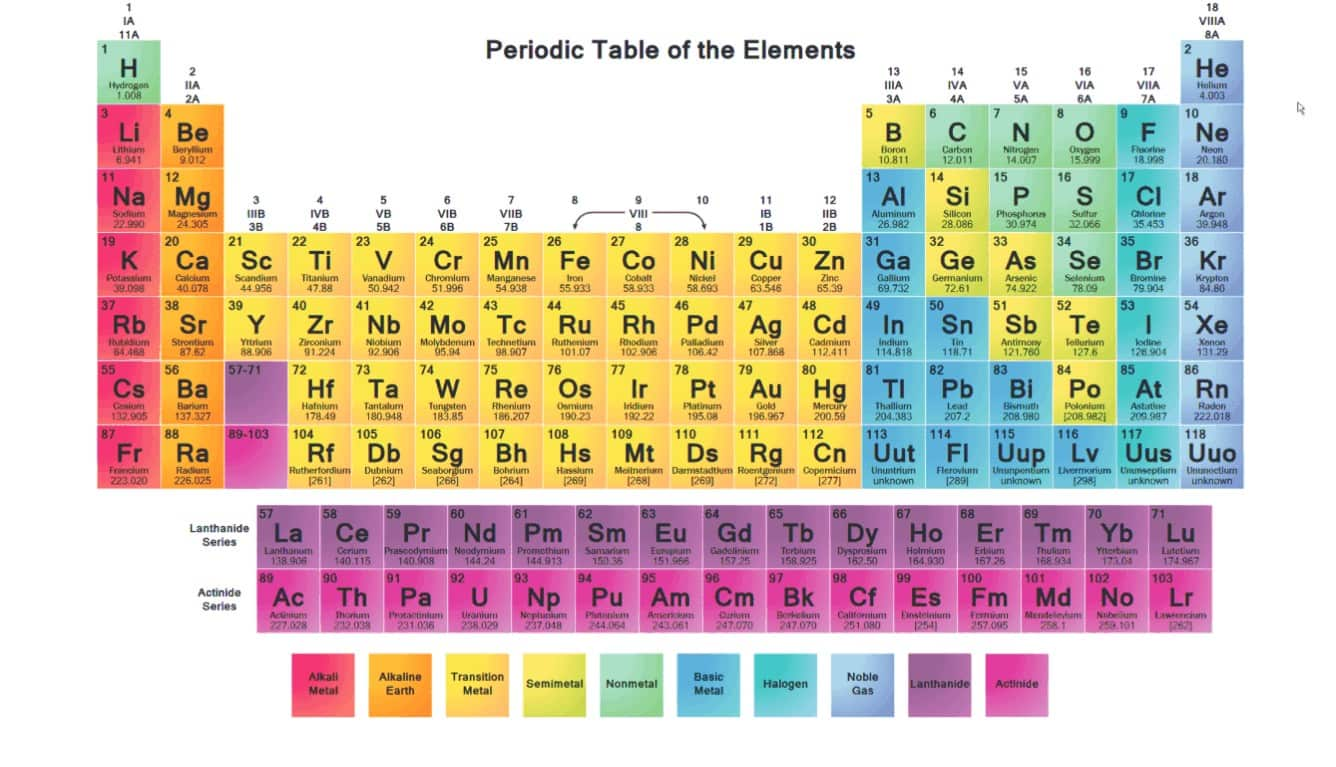
Periodic Table Group 1 Alkali Metals
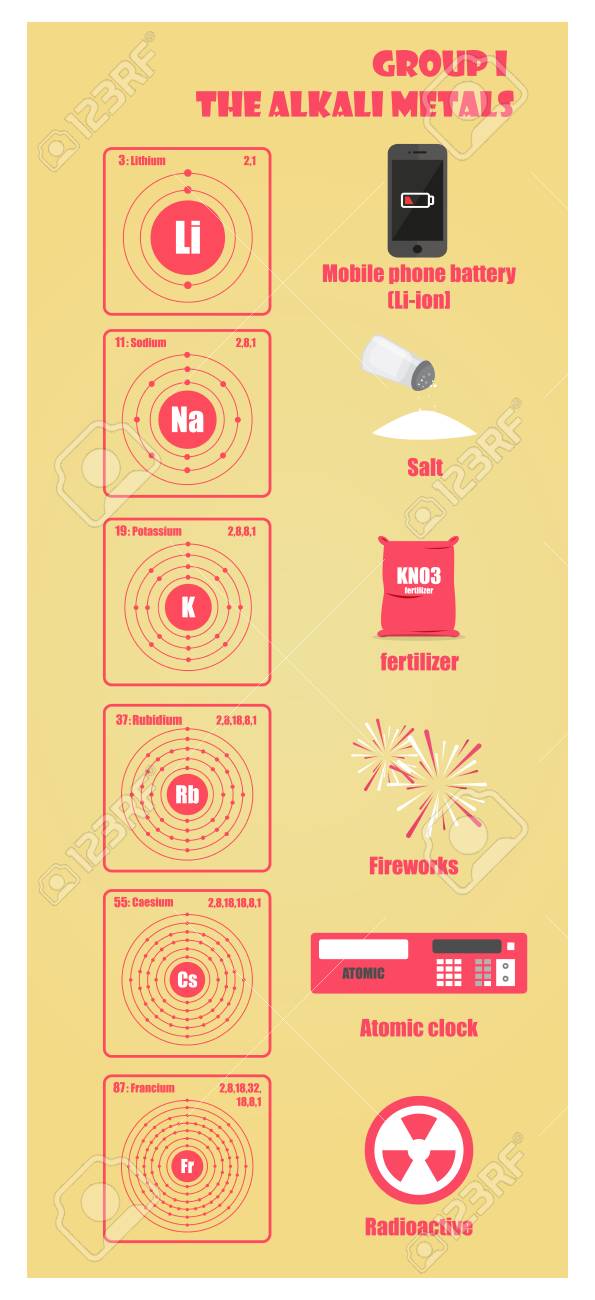
A great summary about group 1 in the periodic table the alkali metals. Alkali metal any of the six chemical elements that make up group 1 ia of the periodic tablenamely lithium li sodium na potassium k rubidium rb cesium cs and francium fr.
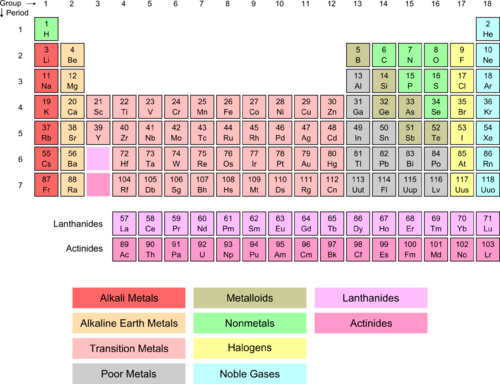
The group 1 elements in the periodic table are known as the alkali metals.
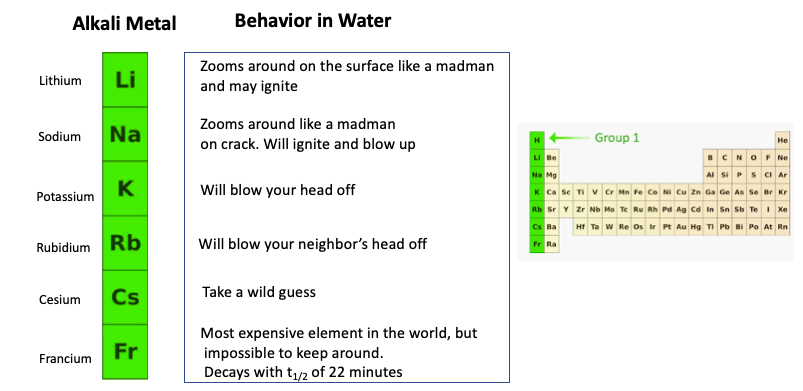
Periodic table group 1 alkali metals. The element hydrogen with one electron per neutral atom is usually placed at the top of group 1 of the periodic table for convenience but hydrogen is not normally considered to be an alkali metal. This family consists of the elements lithium sodium potassium rubidium cesium and francium li na k rb cs and fr respectively. The alkali metals make up group 1 of the periodic table.
In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements. They are all soft silver metals. The alkali metals include.
The f block columns between groups 3 and 4 are not numbered. There are 18 numbered groups in the periodic table. Lithium sodium potassium rubidium cesium and francium.
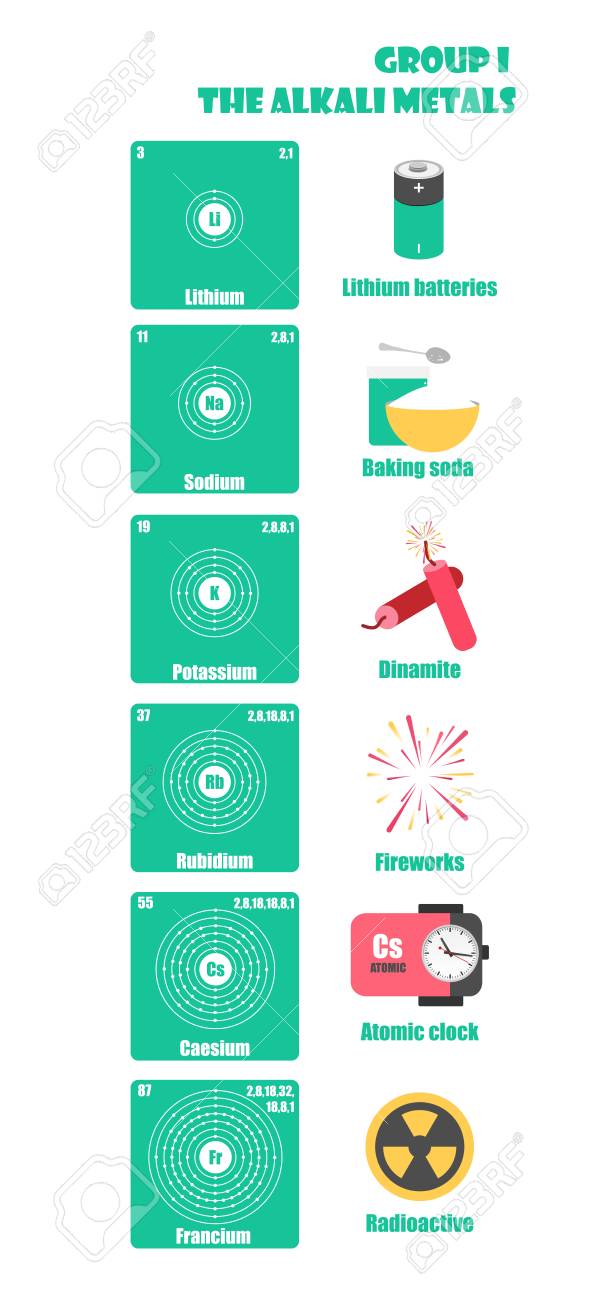
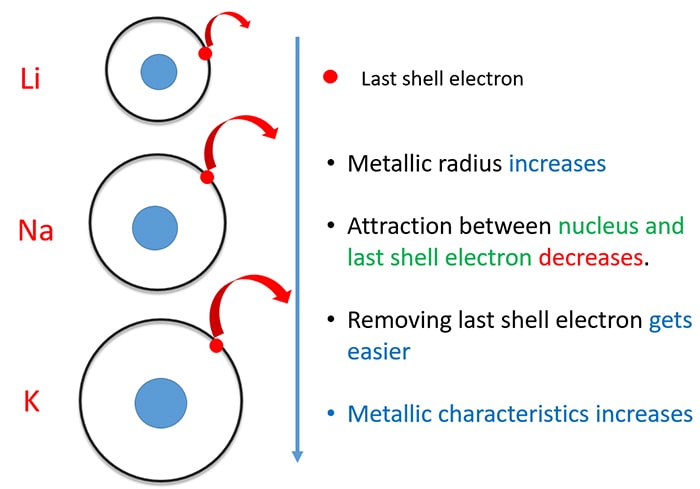
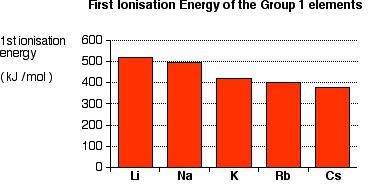
Due to their low ionization energy these metals have low melting. Learn about and revise the alkali metals in group 1 of the periodic table with this bbc bitesize gcse combined science edexcel study guide. Making such classification is sometimes vital to the understanding of different elements.
The group 1 metals are all highly reactive silvery metals that are so reactive to air and moisture that they must be stored under an inert atmosphere or oil. They include lithium sodium and potassium which all react vigorously with water to produce an alkaline solution. At fuse school teachers and animators come together to make fun easy to understand videos in chemistry biology physics.
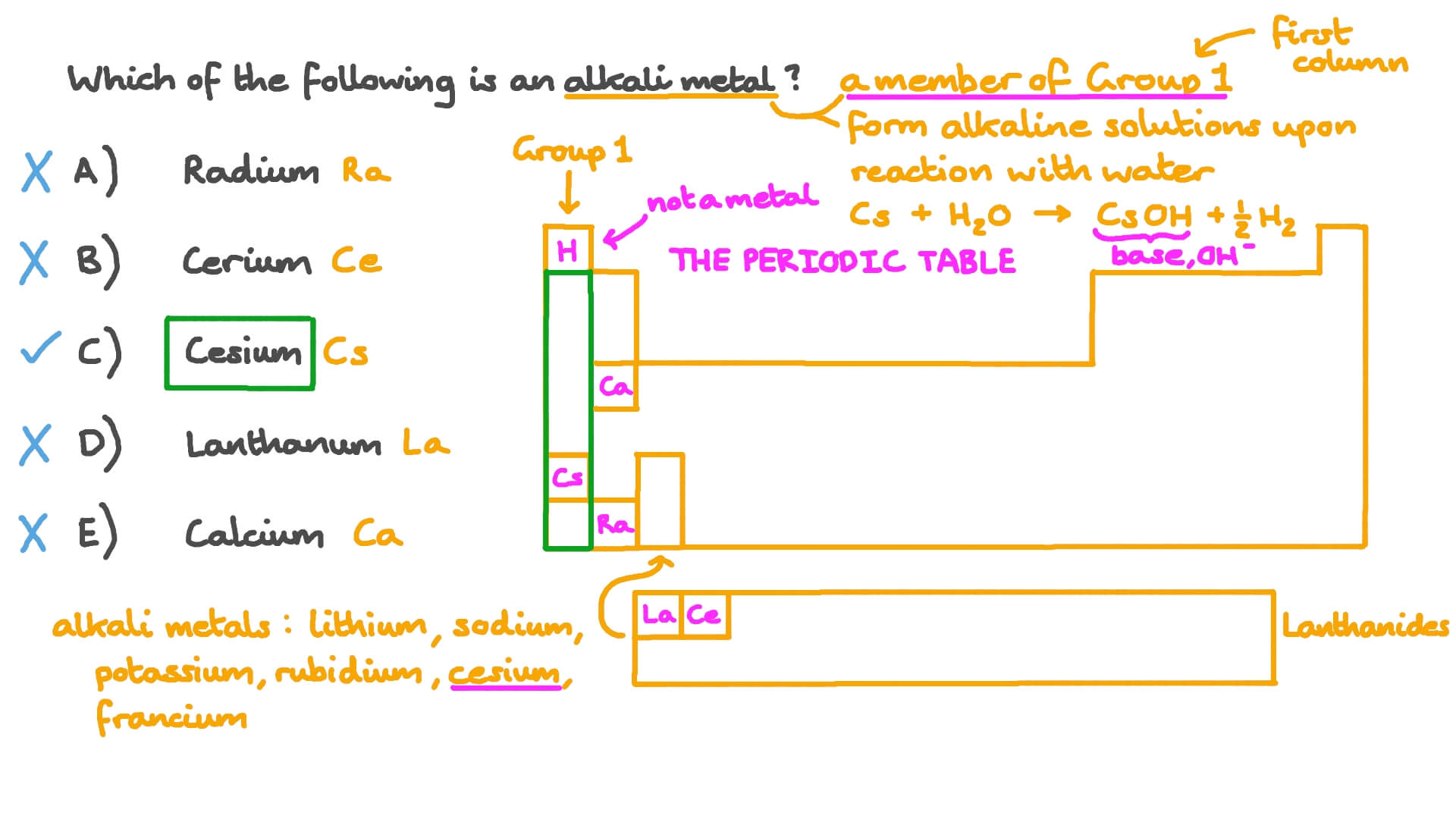
The group 1 elements other than hydrogen are called the alkali metalsthe group 1 elements are. Well depending on the nature of reaction that the metals display some of the metals are called alkali metals. Group one elements share common characteristics.
When it is considered to be an alkali metal it is because of its atomic properties and not its chemical properties. Did you know that the elements in the periodic table are further classified on the basis of their properties. Alkali metals are the chemical elements found in group 1 of the periodic table.
Although often listed in group 1 due to its electronic configuration hydrogen is not technically an alkali metal since it rarely exhibits similar behavior.





























0 Response to "Periodic Table Group 1 Alkali Metals"
Post a Comment