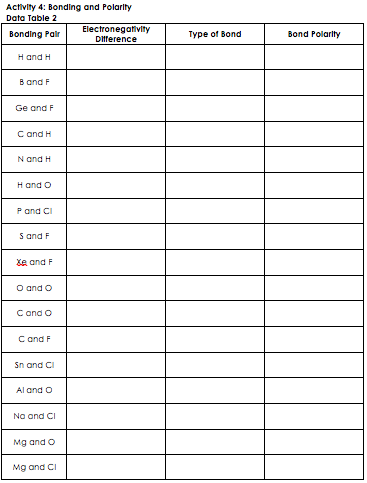
Periodic Table Electronegativity Difference
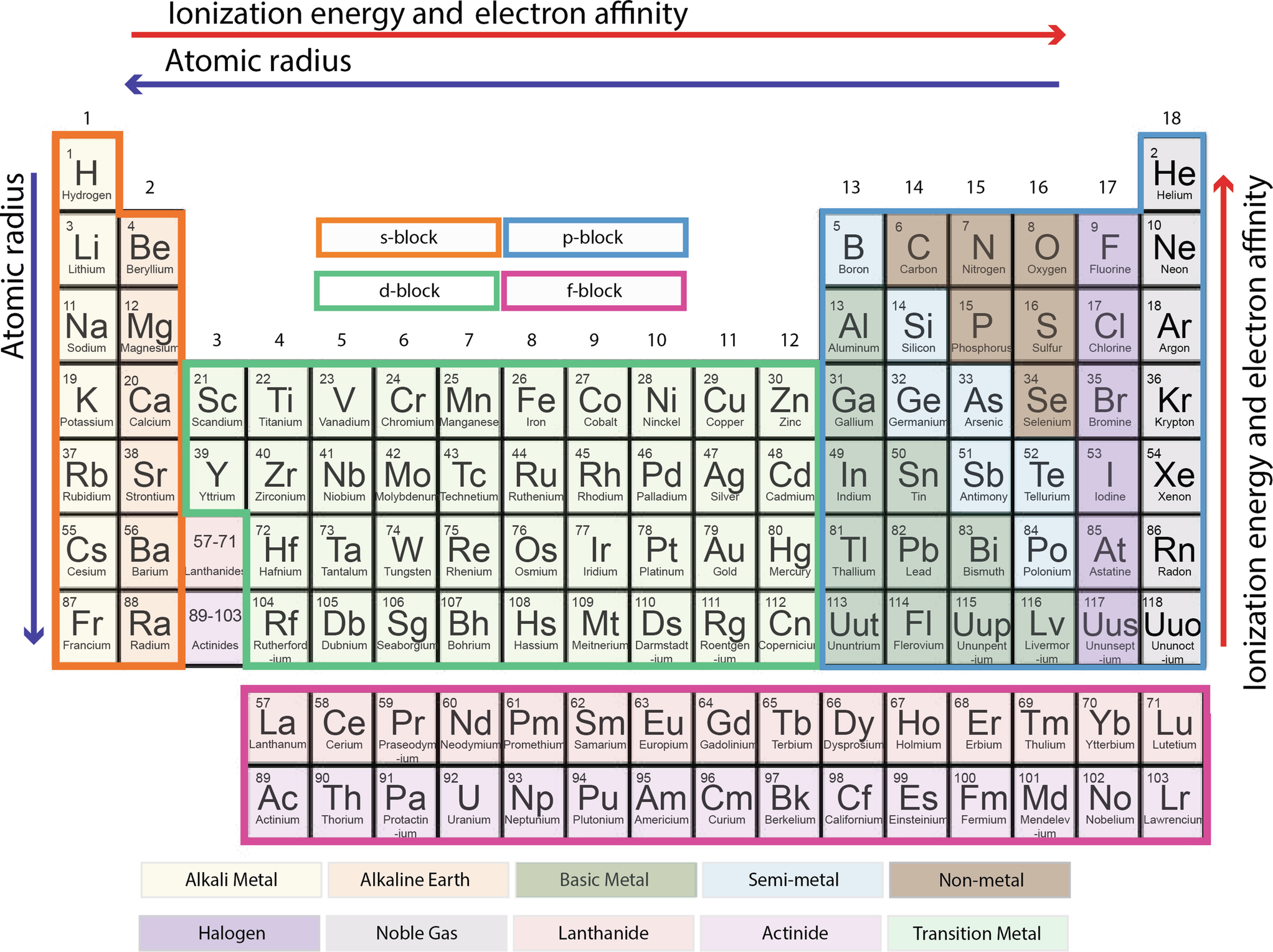
Electronegativity and ionization energy follow the same periodic table trend. This type of bond occurs when there is equal sharing between the two atoms of the electrons in the bond.
So the distance between the nucleus in the outer shell and electrons increases.

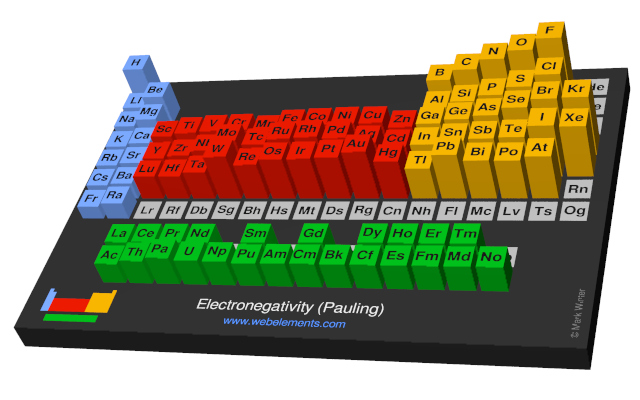
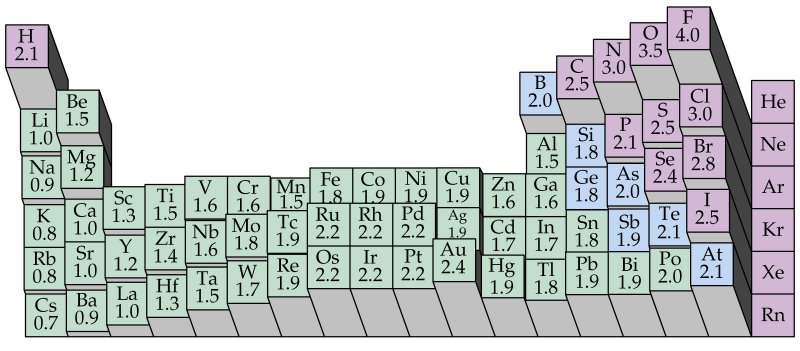
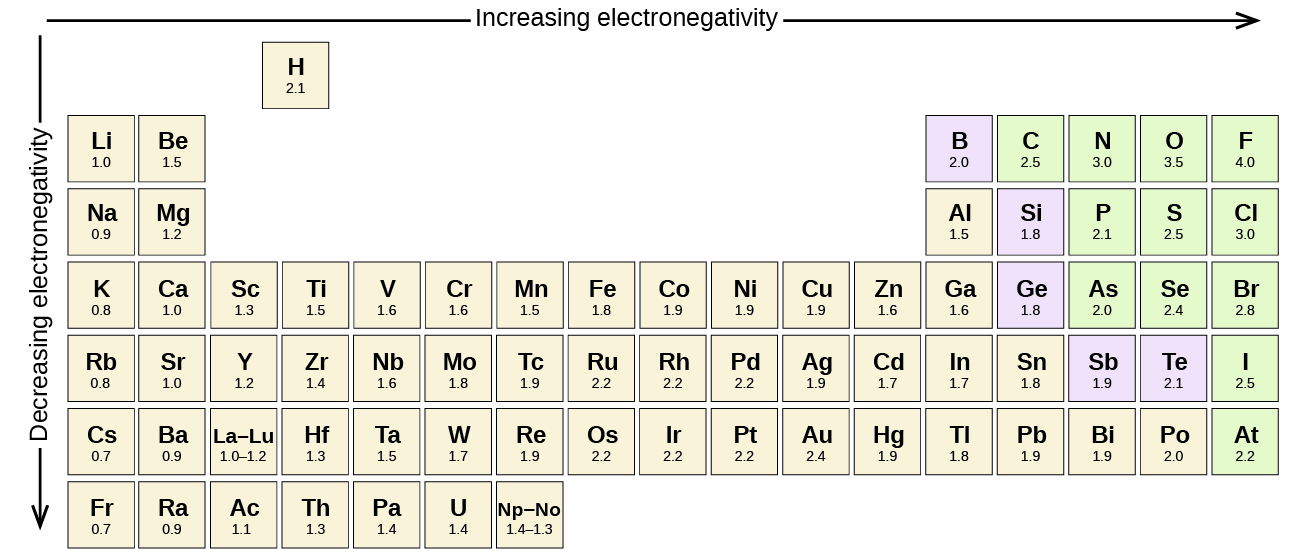
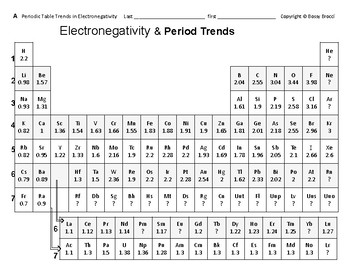
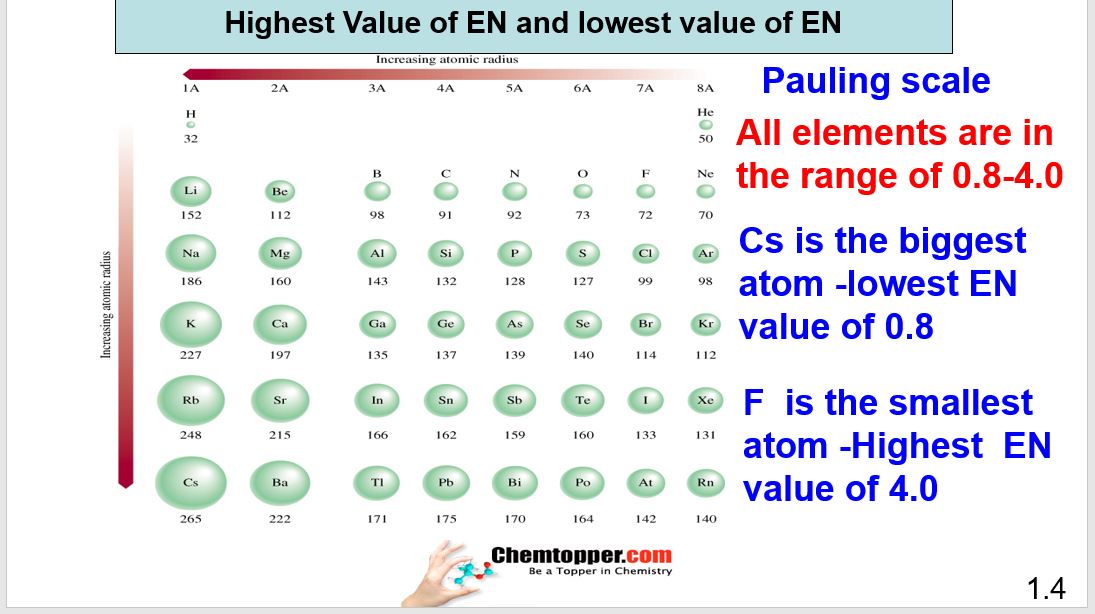
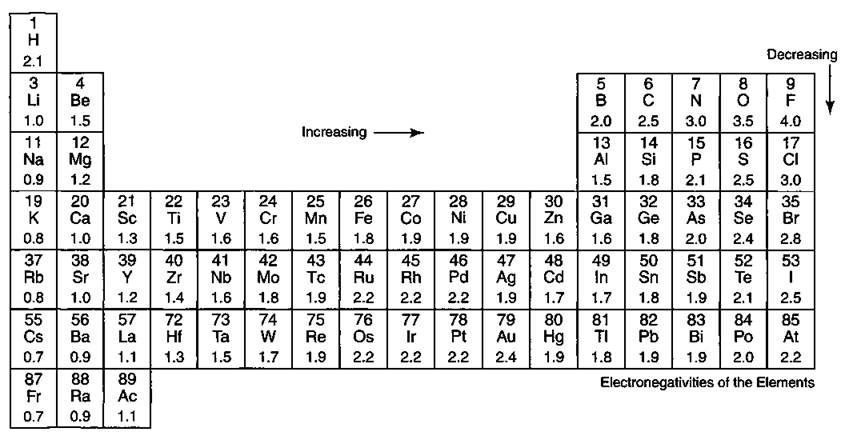
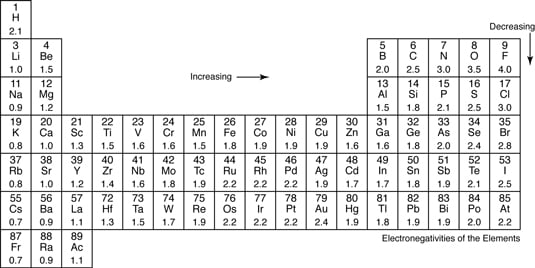
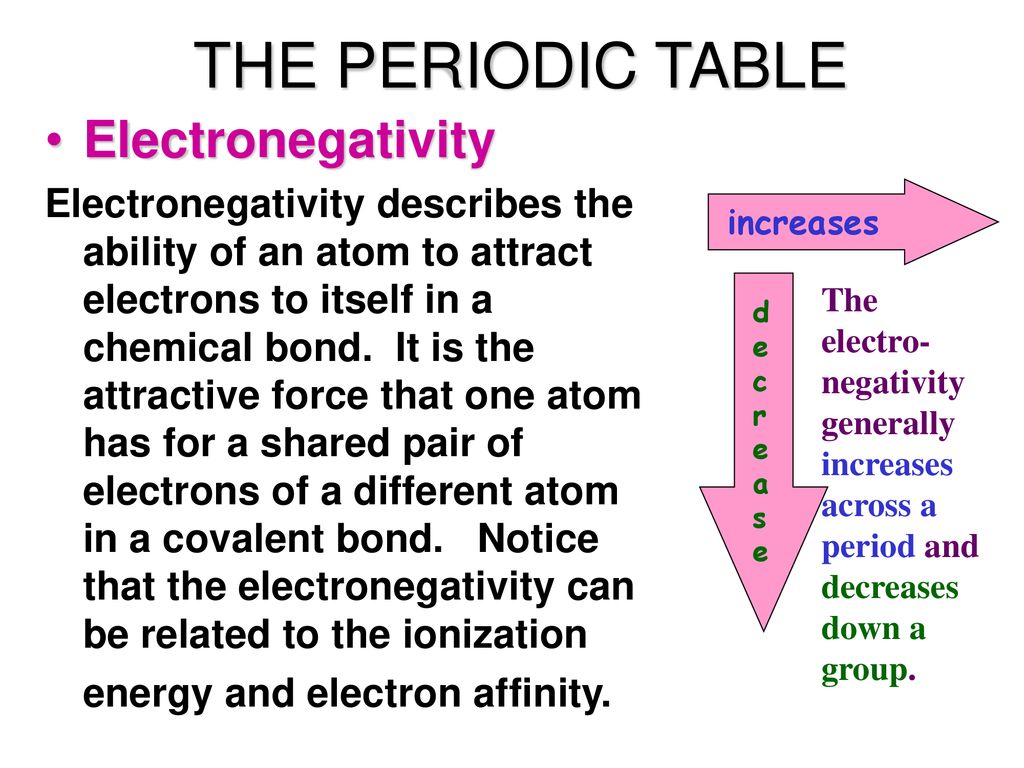
Periodic table electronegativity difference. As you cross the periodic table from right to left electronegativity tends to increase. As you travel down the periodic table from top to bottom the electronegativity decreases. Mass 13 14.
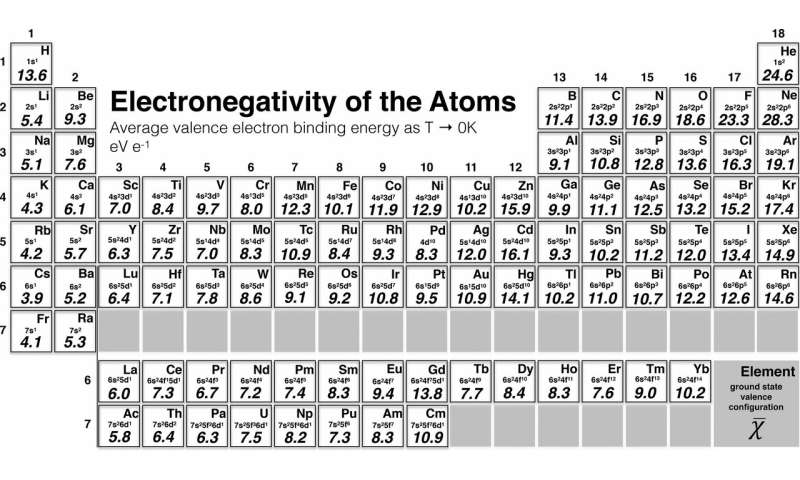
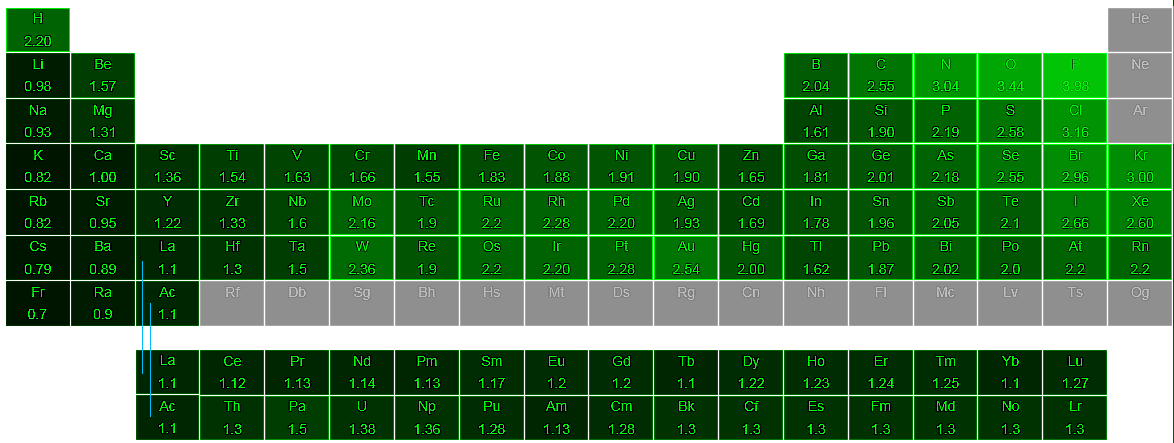
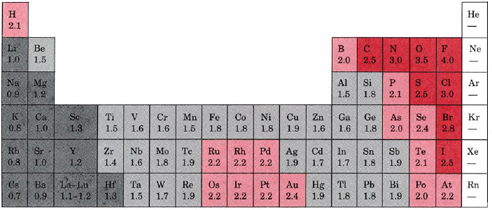
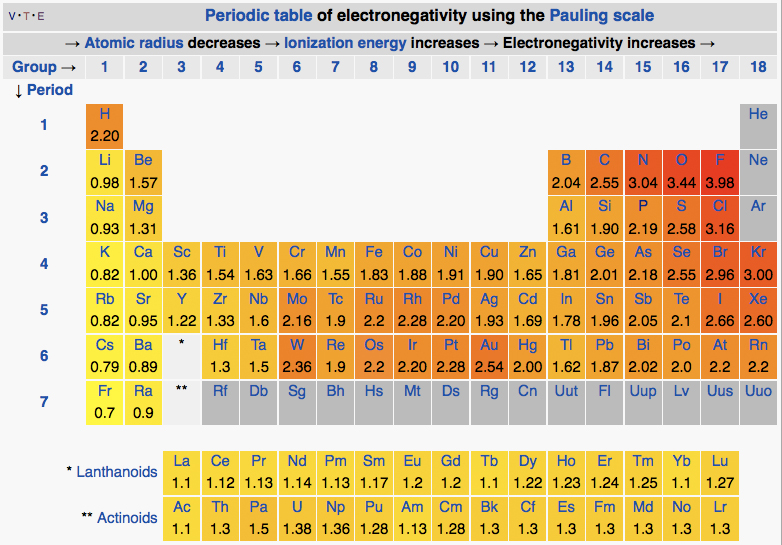
The periodic table of the elements with electronegativities 1 18 hydrogen 1 h 101 21 2 alkali metals alkaline earth metals transition metals lanthanides actinides other metals metalloids semi metal nonmetals 694 halogens noble gases element name 80 symbol beryllium electronegativity mercury hg 20059 19 atomic lithium avg. The nuclei of these atoms dont exert a strong pull on electrons. This simplification ignores the noble gases.
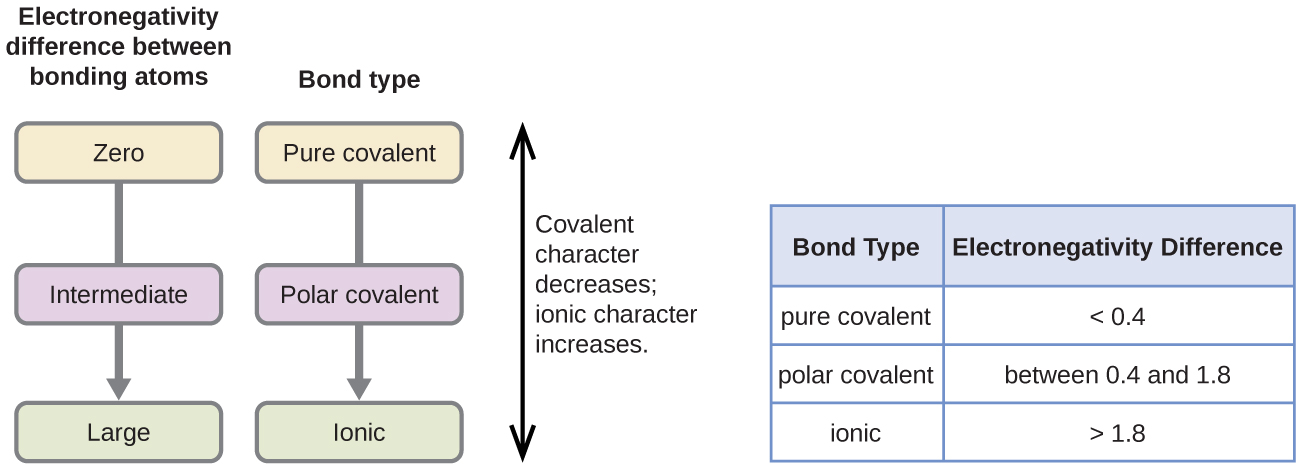
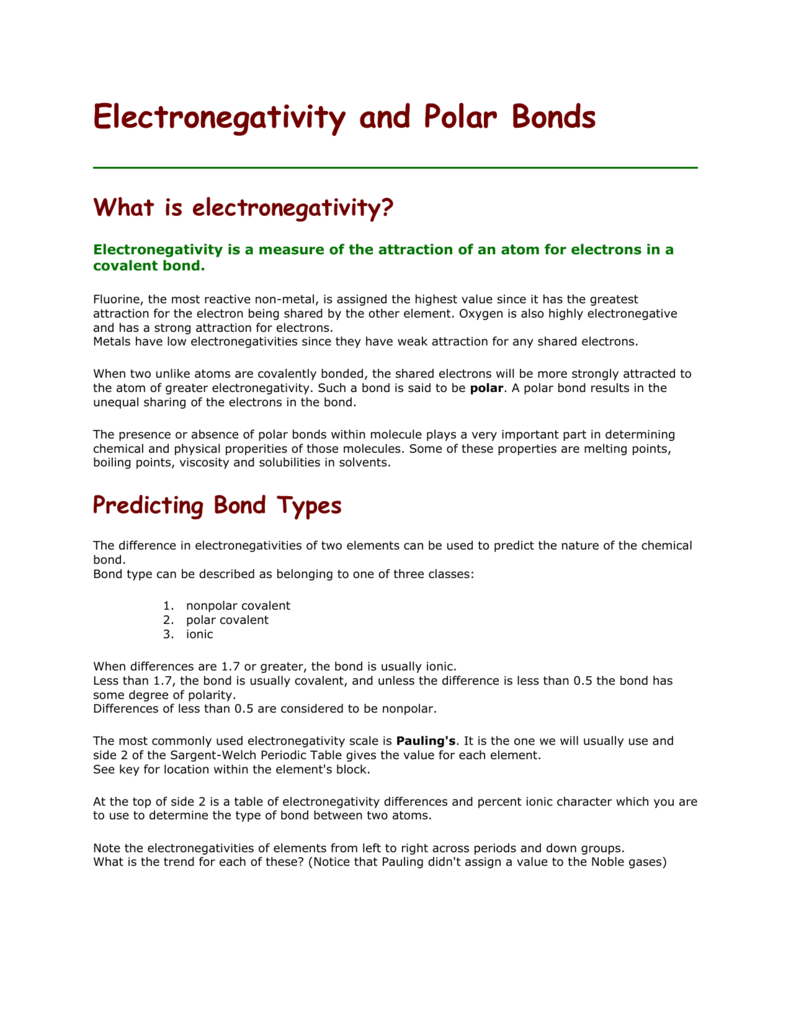
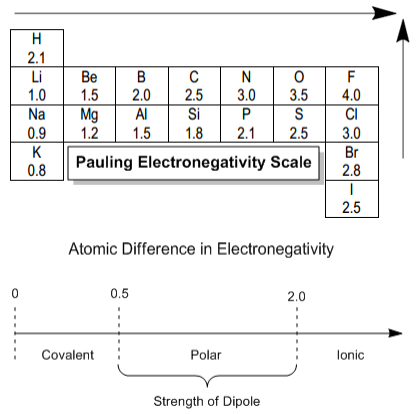
Only the absolute difference is important. Electronegativity trend decreases moving down a periodic table group. In fact the electronegativity difference provides another way of predicting the kind of bond that will form between two elements as indicated in the following table.
Thats an electronegativity difference of 20 30 10 making the bond between the two atoms very very polar. It has been shown that the difference of the electronegativity chart will vary with each one of the elements environment. Therefore electronegativity decreases moving down a group in a periodic table.
The greater an atoms electronegativity the greater its ability to attract electrons to itself. And also the effective nuclear charge remains the same moving down a periodic table group. Elements that have low ionization energies tend to have low electronegativities.
The difference between electronegativities can be used to determine the. Its monatomic form h is the most abundant chemical substance in the universe constituting roughly 75 of all baryonic mass. Additionally it has also been discovered that atoms that usually have higher.
Keep in mind that electronegativities are approximate measures of the relative tendencies of these elements to attract electrons to themselves in a chemical bond. Similarly elements that have high ionization energies tend to have high electronegativity values. Atoms with low electronegativity are more likely to have their bonding electrons pulled away.
Calculate the difference between their electronegativity values. When moving down a periodic table group the atomic radius increases due to the increasing number of shells. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structurethe chemical symbol for hydrogen is h.
If you remember that fact everything becomes easy because electronegativity must always increase towards fluorine in the periodic table. Only the absolute difference is important. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
Historically this is because they were believed not to form bonds and if they dont form bonds they cant have an electronegativity value. The truth is that the pauling scale helps you determine or calculate the electronegativity of a single element in the periodic table. The atomic nucleus exerts a strong pull on electrons.
















/PeriodicTableElectronegativity-56a12a045f9b58b7d0bca77c.jpg)



0 Response to "Periodic Table Electronegativity Difference"
Post a Comment