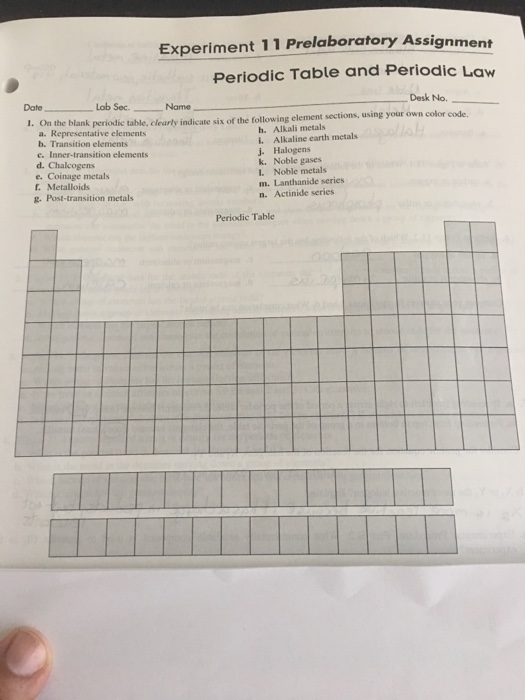
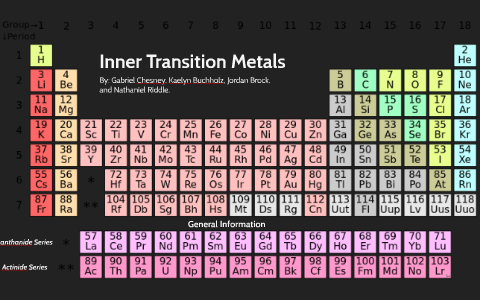
Periodic Table Of Elements Inner Transition Metals
There are 35 elements located in the d block. These are subcategorized by two individual series called the lanthanoids and actinoids and they occupy.
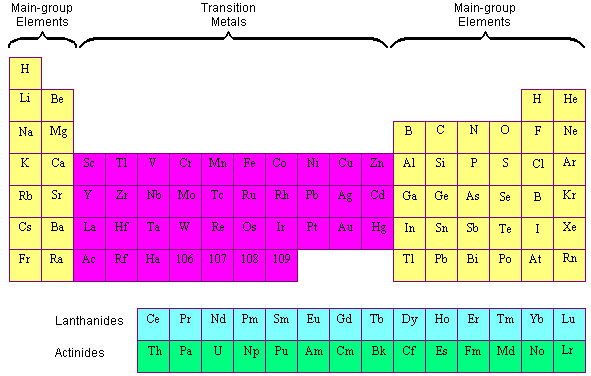
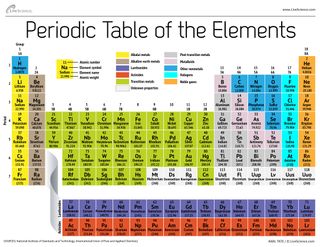
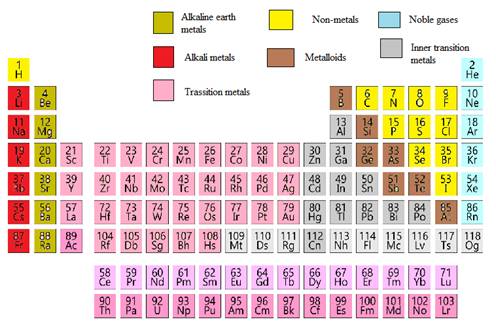
Many scientists describe a transition metal as any element in the d block of the periodic table which includes groups 3 to 12 on the periodic table.
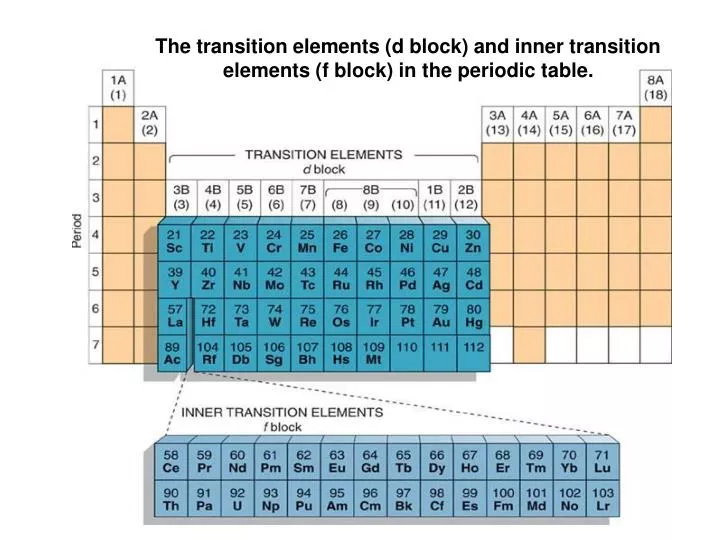
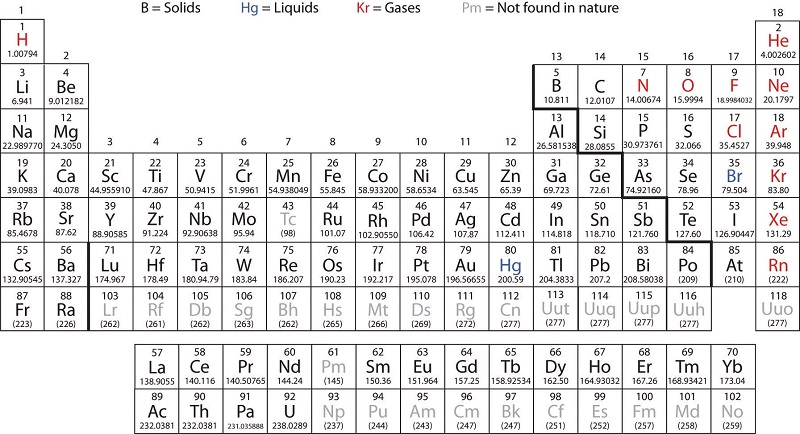
Periodic table of elements inner transition metals. Helium is an s element but nearly always finds its place to the far right in group 18 above the p element neon. The actinides are all radioactive. They include elements 57 71 known as lanthanides and 89 103 actinides.
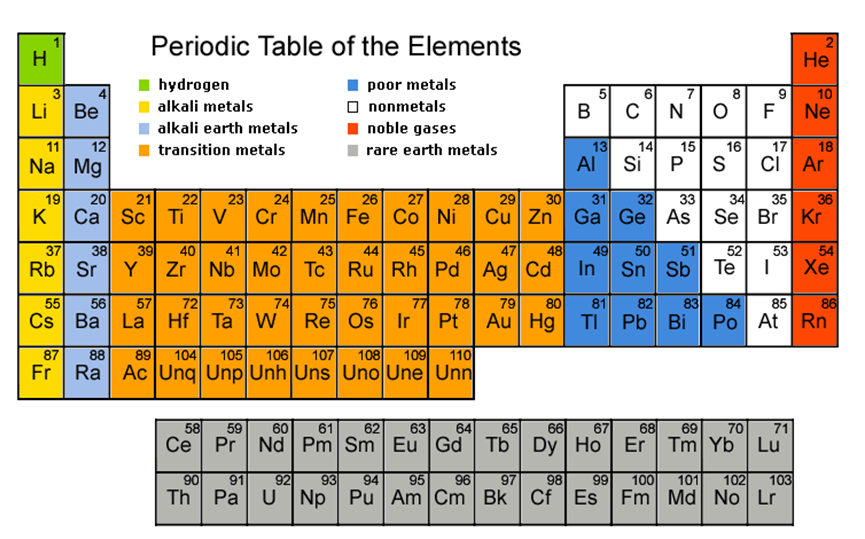
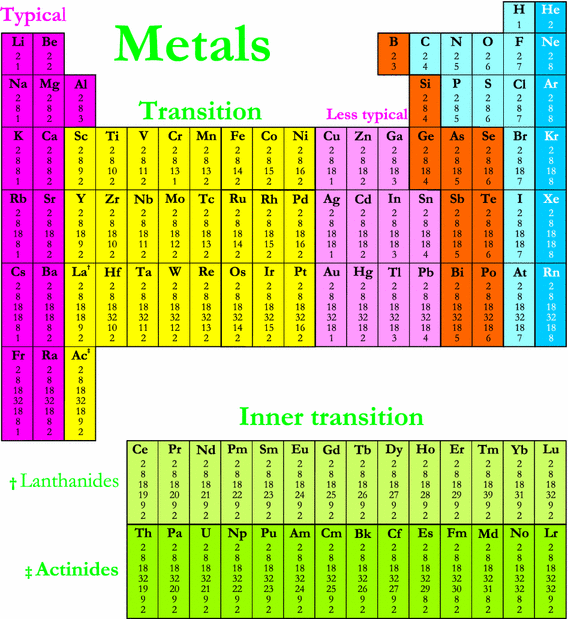
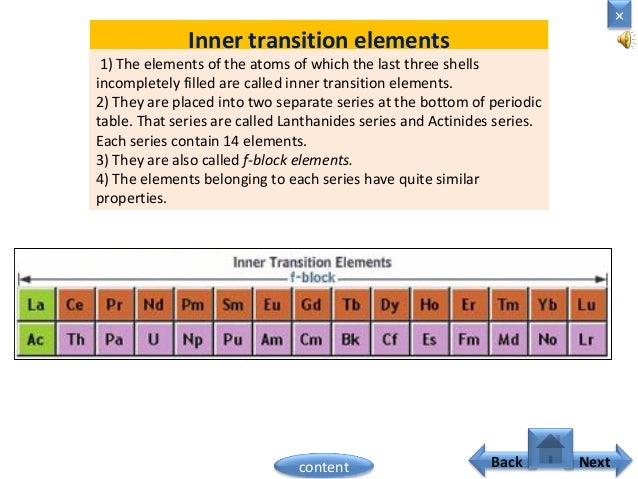
In actual practice the f block lanthanide and actinide series are also considered transition metals and are called inner transition metals. They are normally shown in two rows below all the other elements. In this article we are going to read about two periods in the periodic table which occupy 30 chemical elements and are called the inner transition metals.
The name transition metal refers to the position in the periodic table of elements. Their general valence configuration is ns 12. Sometimes the elements of column twelve of the periodic table zinc cadmium mercury copernicium are not included as part of the transition metal group.
Lanthanides are located inperiod 6. The lanthanides are very similar. There are 35 elements located in the d block.
4f and 5f orbitals of f block elements are steadily in later of two long periods. It is based on these periods and groups that the 103 elements in nature are classified. D and f block elements in the groups of 3 to 11 are also called as transition elements and inner transition elements respectively.
Based on this they are differentiated in lanthanides and actinides. Elements 58 71 which follow lanthanum are the lanthanides and elements 90 103 which follow actinium are the actinides. Actinides are located inperiod 7.
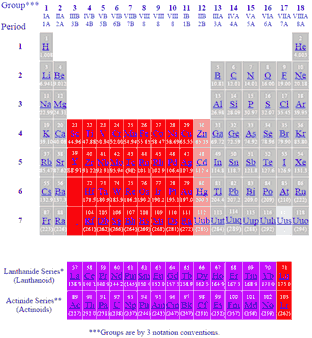
Also the two rows of elements below the main body of the periodic table the lanthanides and actinides are special subsets of these metals. In this way the transition metals represent the transition between group 2 2a elements and group 13 3a elements. The transition elements represent the successive addition of electrons to the d atomic orbitals of the atoms.
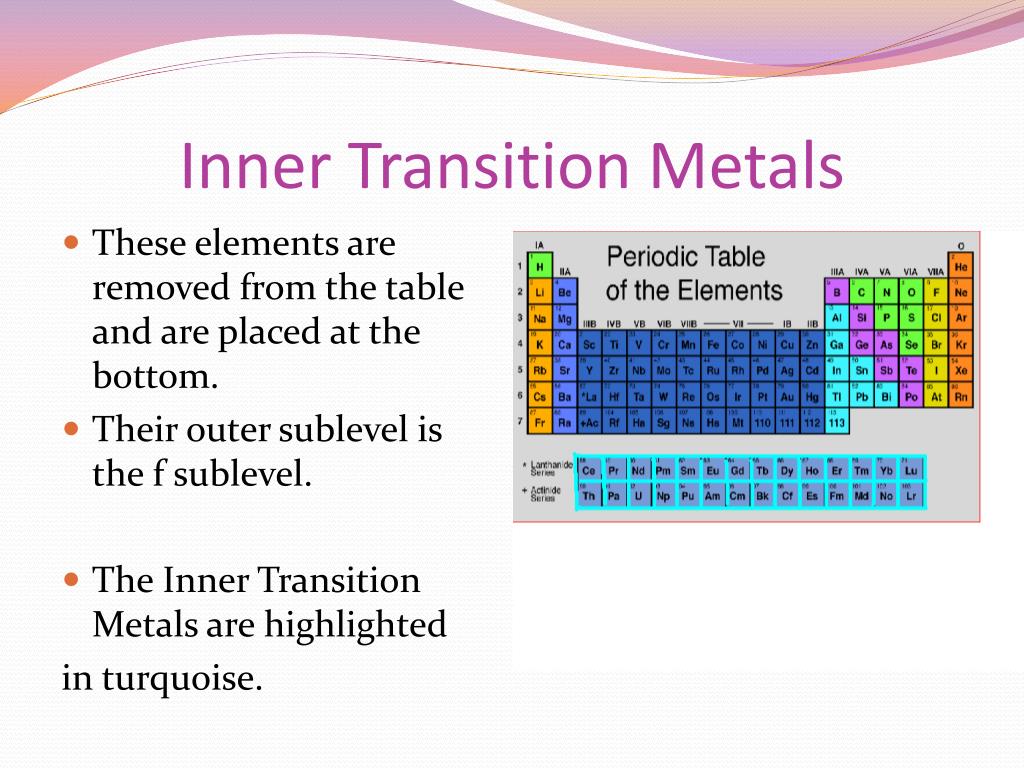
The inner transition metals are shown in two rows at the bottom in pink and purple an inner transition metal itm of chemical elements on the periodic table. The inner transition elements occupy a position in between the elements lanthanum z57 and hafnium z72 and between actinium z89 and rutherfordium z104. The s block is on the left side of the conventional periodic table and is composed of elements from the first two columns the nonmetals hydrogen and helium and the alkali metals in group 1 and alkaline earth metals group 2.
The largest group of elements on the periodic table is that of the transition metals which is found in the middle of the table. These elements were sometimes called rare earth elements or rare earth metals due to their extremely low natural occurrence. The transition metal group is called the d block of the periodic table.






















0 Response to "Periodic Table Of Elements Inner Transition Metals"
Post a Comment