Periodic Table Iron And Nickel
Nickel periodic table. Its symbol is ni and its atomic number is 28.
Members of a group typically have similar properties and electron configurations in their outer shell.

Periodic table iron and nickel. The term has different meanings in different contexts. In addition it lies at the top of group 8 former group 8b. The atomic number of each element increases by one reading from left to right.
It has 28 protons and 28 electrons in the atomic structure. Periodic table of elements and x ray energies innovation with integrity handheld xrf 1 101 h 00007 hydrogen 2 400 he00002 helium 3 694 li 053 lithium 4 901 be 185 beryllium ka 0108. In chemistry and physics the iron group refers to elements that are in some way related to iron.
Iron fe cobalt co and nickel ni which share similar chemical and physical characteristics. The atomic number of each element increases by one reading from left to right. Our periodic element comparison tool allows you to compare periodic elements properties side by side for all 118 elements schoolmykids interactive dynamic periodic table periodic table element comparison tool element property trends.
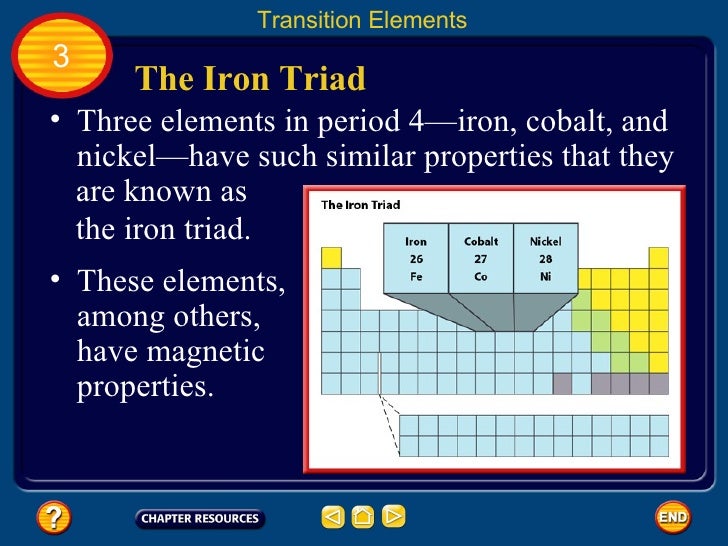
A vertical column in the periodic table. In chemistry the term is largely obsolete but it often means iron cobalt and nickel also called the iron triad. Chemical element in the periodic table of elements.
It is located in period 4 of the periodic table situated between manganese and cobalt. Its atomic weight is 5869. In chemical terms it is classified as a transition metal.
The iron triad is composed of three elements. Nickel is a 28. The chemical symbol for nickel is ni.
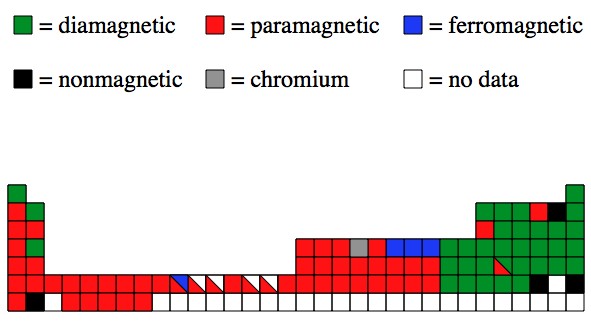
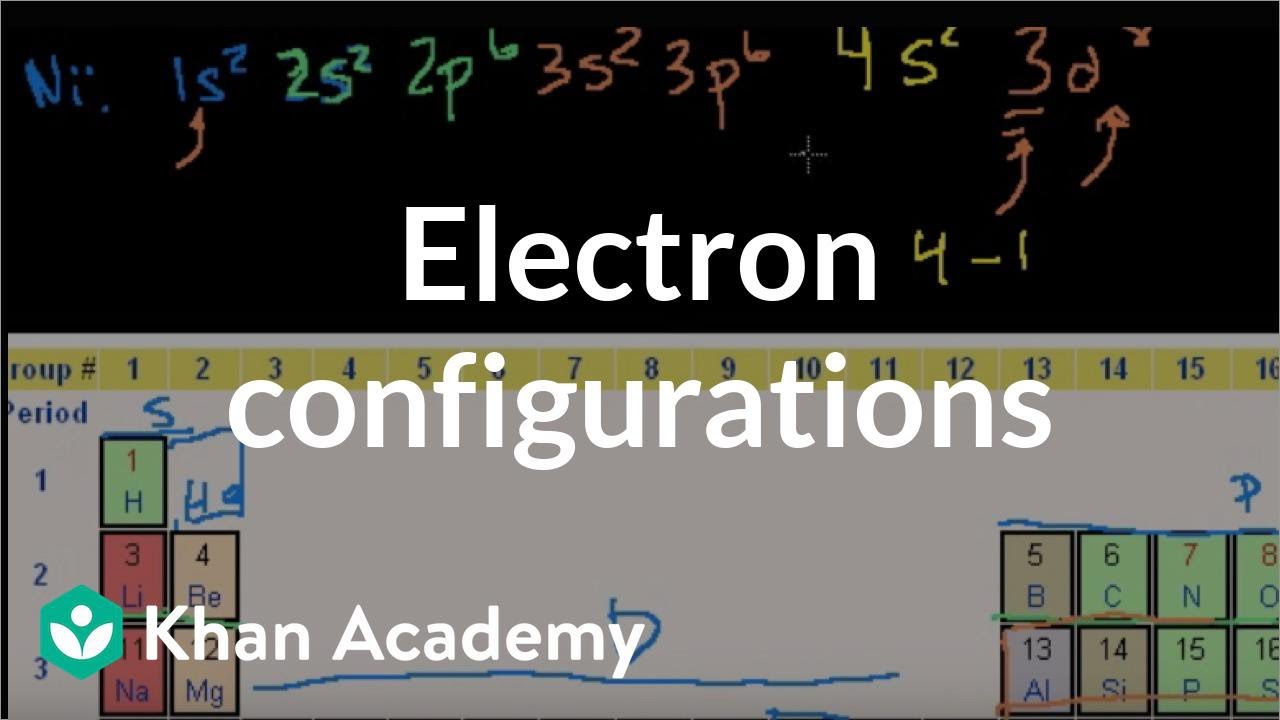
Iron cobalt and nickel have a number of similar properties and were once grouped together as group 8b 4. Mostly in period row 4 of the periodic table. These electrons can move between the d and s shells to give you different valences and oxidation states.
Nickel curie temperature is 3550c which means above this temperature nickel becomes non magnetic. For example here are three ways that the electrons can occupy the 4s and 3d orbitals for iro. Iron has the ability to form.
Members of a group typically have similar properties and electron configurations in their outer shell. They are found adjacent to each other in period 4 of the periodic table. Its density is 890 gcm 3 at 25 0 c.
Period a horizontal row in the periodic table. Nickel belongs to transition metals in periodic table. A vertical column in the periodic table.
In iron and nickel you have 8 valence electrons. In combination with iron nickel is. Compare iron vs nickel of the periodic table on all their facts electronic configuration chemical physical atomic properties.
Period a horizontal row in the periodic table. Or sometimes other elements that resemble iron in some chemical aspects.

















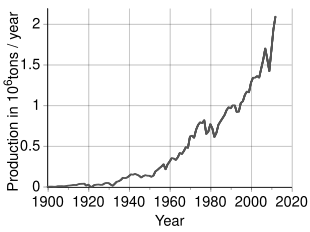

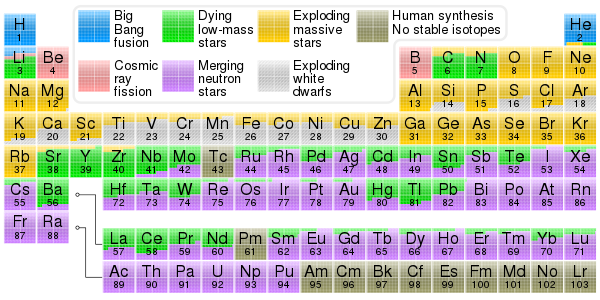


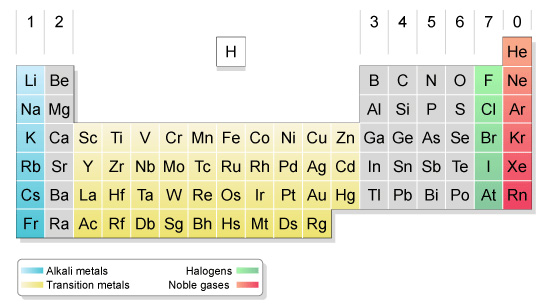










0 Response to "Periodic Table Iron And Nickel"
Post a Comment