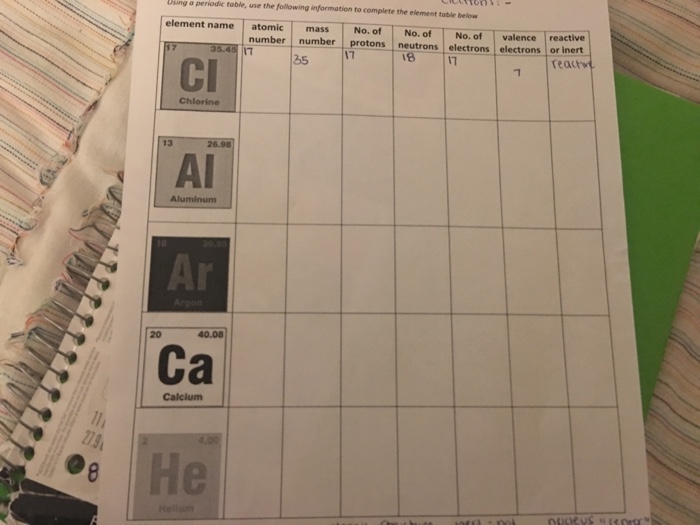
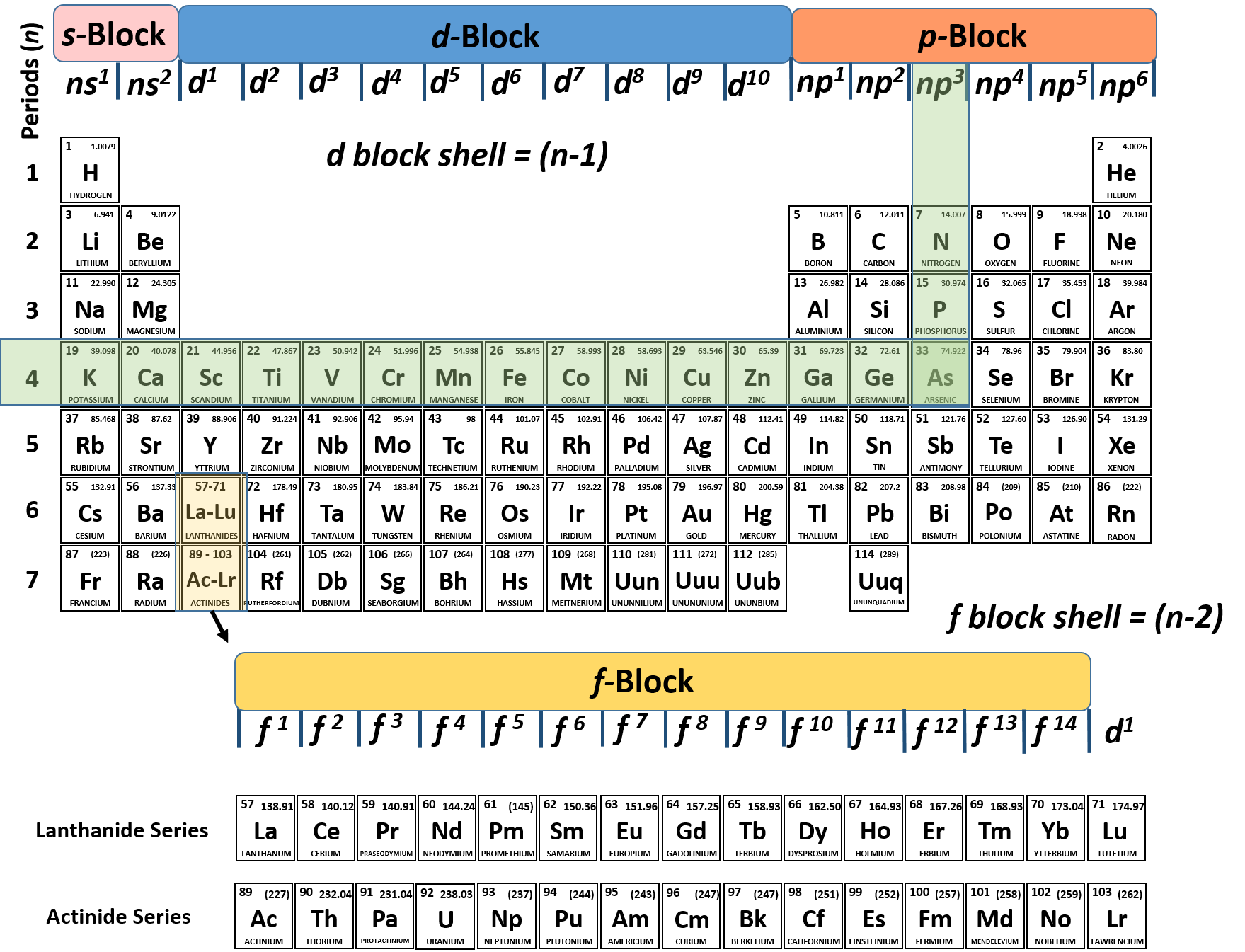
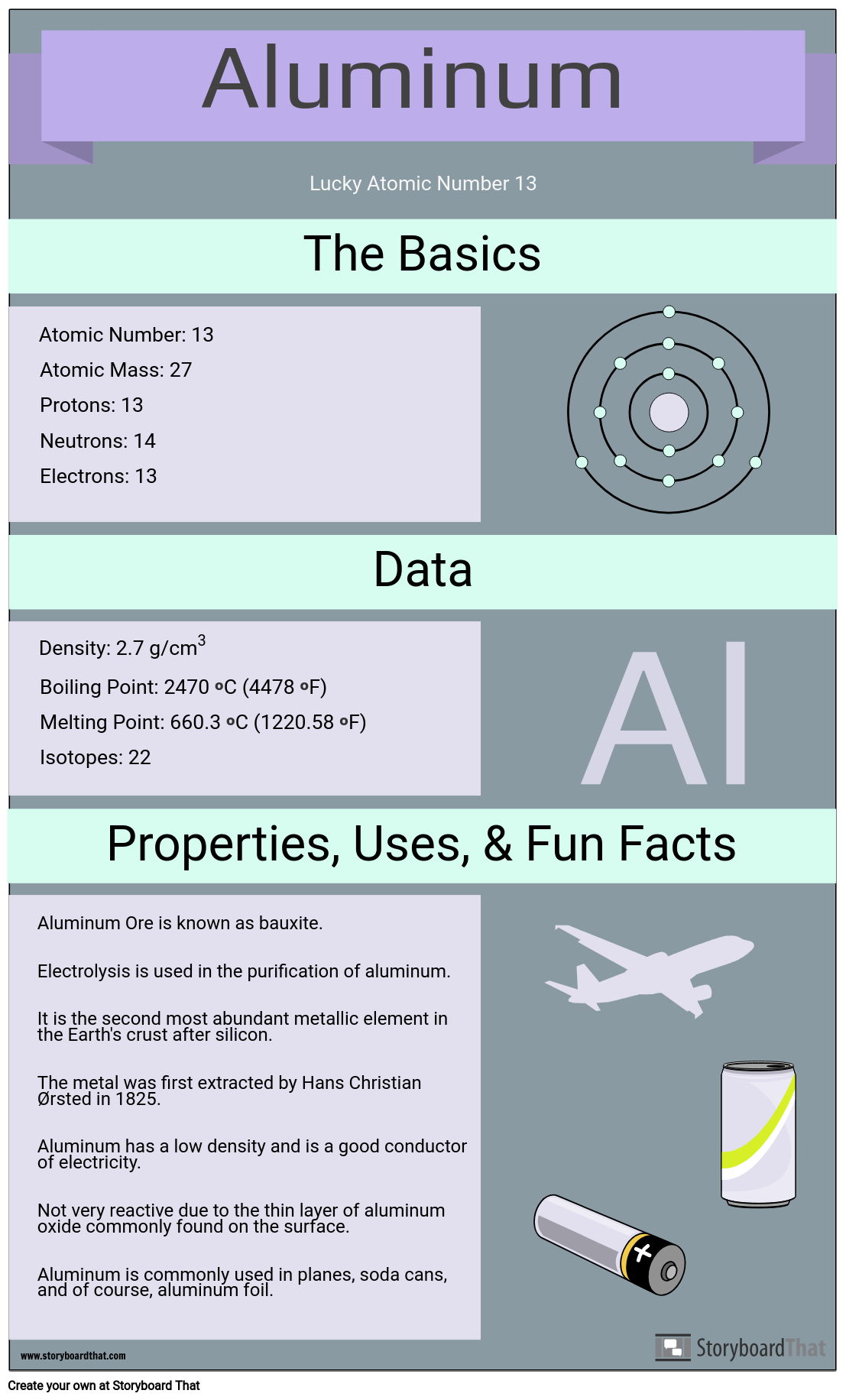
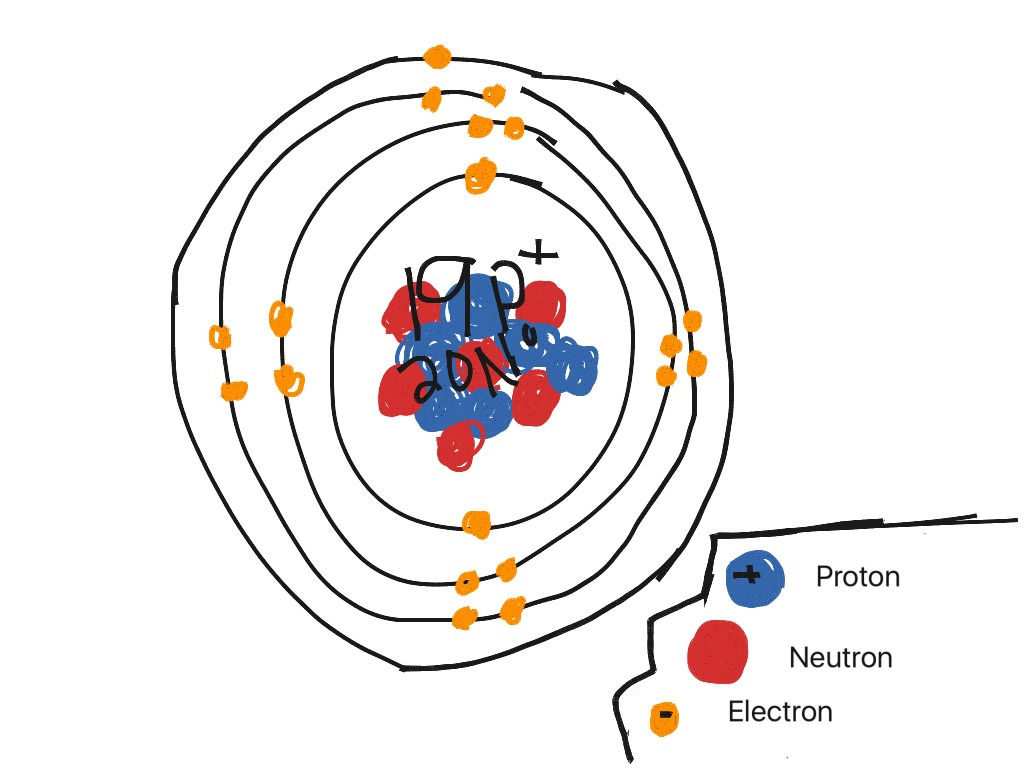
Periodic Table Aluminum Protons Neutrons Electrons
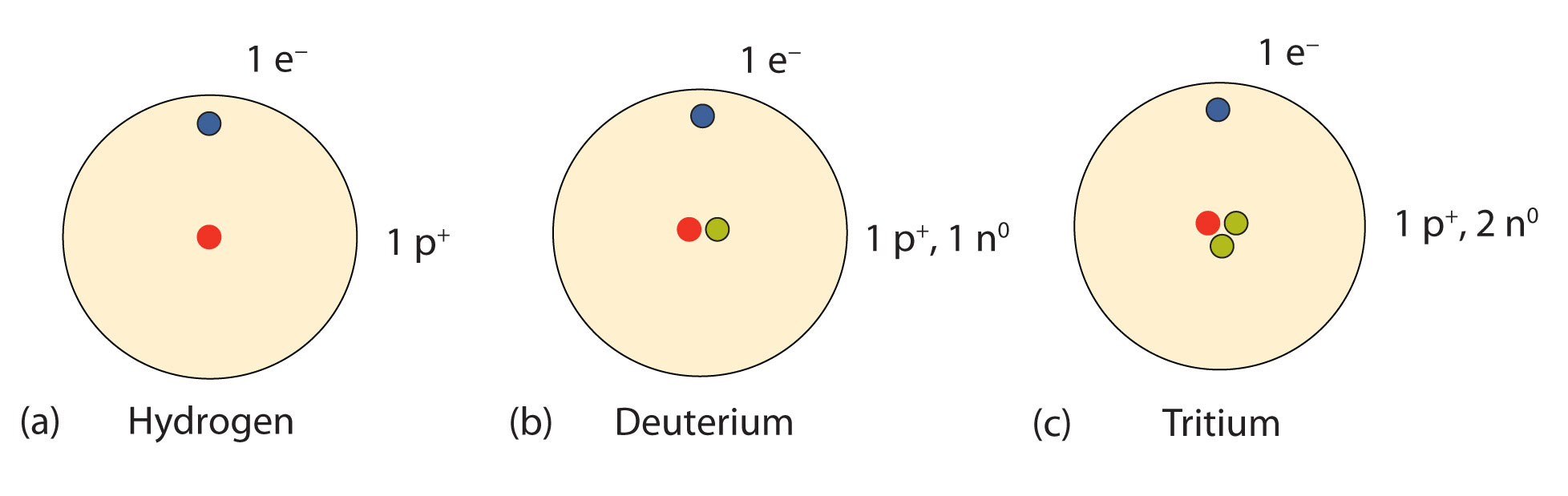
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structurethe chemical symbol for hydrogen is h.
Also there will be 13 electrons in a neutral atom of this poor metal.
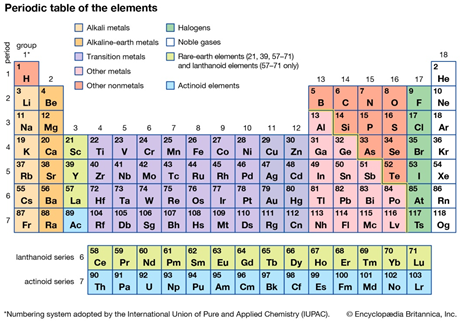
Periodic table aluminum protons neutrons electrons. Electrons surround the nucleus. It does not rust and doesnt easily react to weather conditions or chemicals. Alkali metals alkaline earth metals transition metals other metals metalloids non metals halogens noble gases rare earth elements.
Name atomic number atomic mass electron configuration number of neutrons melting point boiling point date of discovery crystal structure. Under these circumstances it will have 10 11 or 12 electrons. Atoms of aluminum that are involved in chemical bonds will have 10 11 or 12.
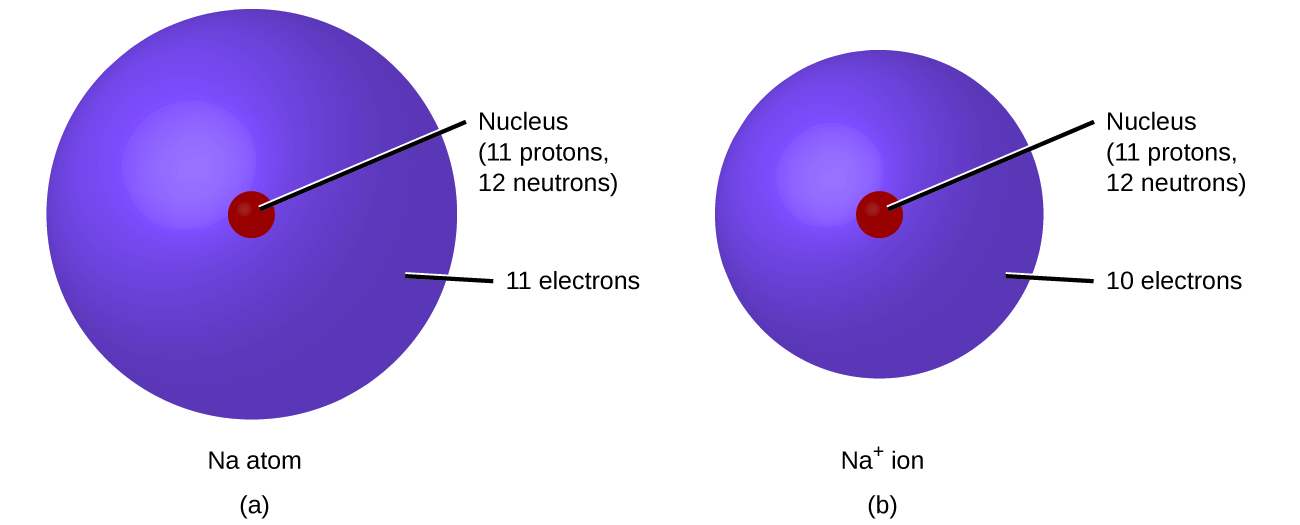
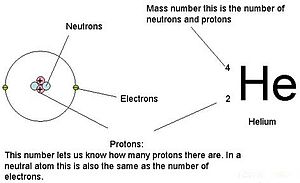
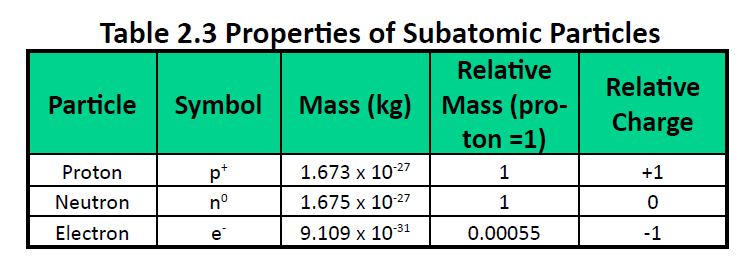
Protons have a positive charge. Aluminum periodic table protons neutrons and electrons. Atoms are made of extremely tiny particles called protons neutrons and electrons.
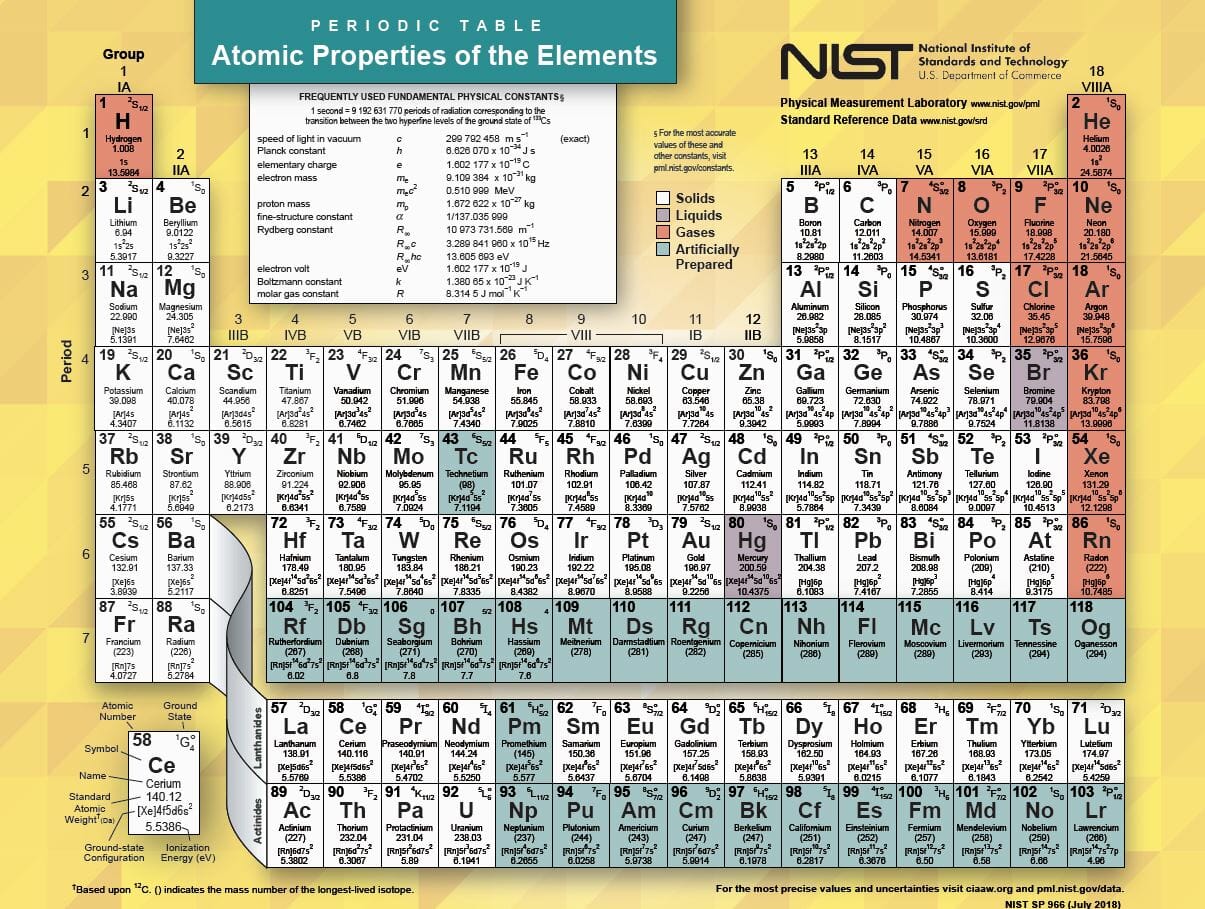
Oftentimes part of your answer will be right in front of you in the periodic table. The number of neutrons in an aluminum atom depends on the isotope. Potassium aluminum sulfate kalso 4 2 12h 2 o interesting facts.
Its the third most common of all the elements found in the earths crust oxygen and silicon being found more. The element aluminum has 13 protons. Finding the number of protons neutrons and electrons in a given element isnt as hard as it sounds.
Its monatomic form h is the most abundant chemical substance in the universe constituting roughly 75 of all baryonic mass. There are 13 protons in the element aluminum. How many protons neutrons and electrons are present in an atom of bohr rutherford diagrams for neutral atoms ppt online chemical elements com aluminum al aluminum number of protons.
How many protons neutrons and electrons are present in an atom of chemical elements com aluminum al aluminum number of protons atomic structure periodic table review ppt online. Its never found free in its natural state. Protons and neutrons are in the center of the atom making up the nucleus.
The most common and natural isotopes are al27 and al26 thus these atoms have 14 and 13 neutrons respectively isotopes of aluminium. The charge on the proton and electron are exactly the same size but opposite. Al has the atomic number 13 that is it has 13 protons in the atom aluminium.
Whats people lookup in this blog. Whats people lookup in this blog. It will have 13 electrons in its neutral state though aluminum loans out electrons to form bonds.
Once you know where to look finding the number of protons neutrons and electrons will be a breeze. Aluminum periodic table protons neutrons electrons. Aluminum oxide al 2 o 3.
Electrons have a negative charge.









:max_bytes(150000):strip_icc()/what-are-the-first-20-elements-608820-FINAL-5b758ab446e0fb002c67279a.png)













0 Response to "Periodic Table Aluminum Protons Neutrons Electrons"
Post a Comment