Periodic Table Mass Units
Since c13 and c14 are both heavier than c12 the average is a bit higher than you expected. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search.
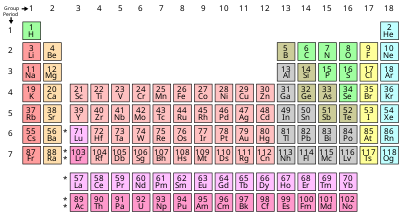
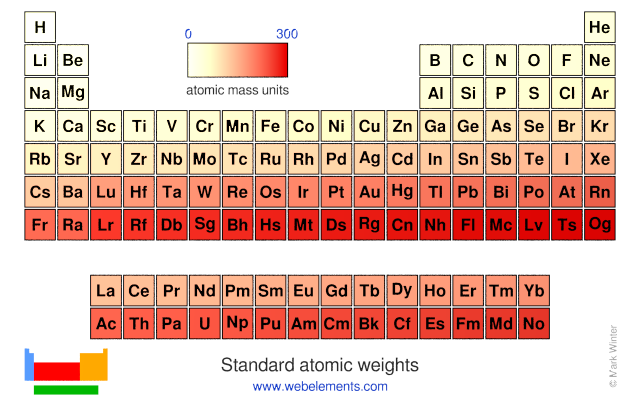
Chemical elements listed by atomic mass the elemenents of the periodic table sorted by atomic mass.

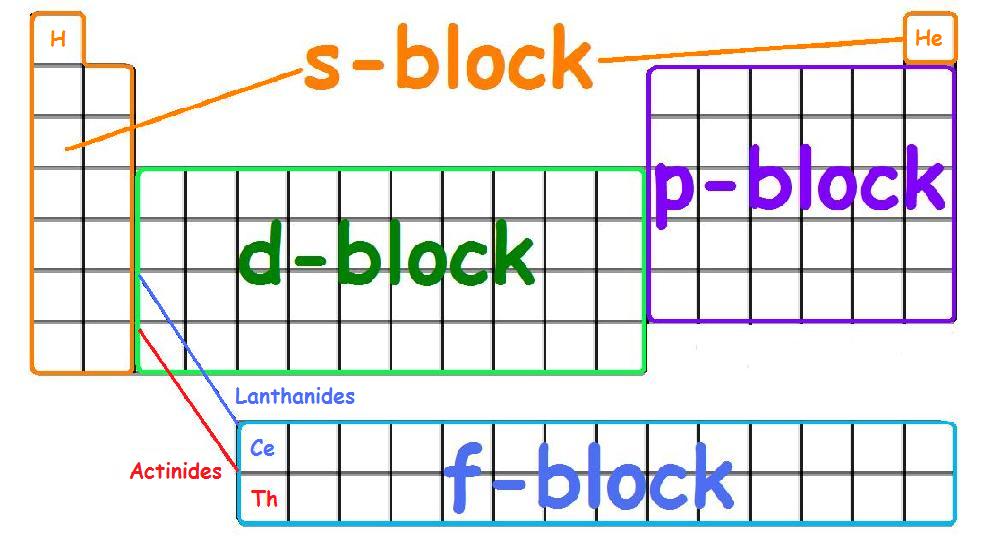
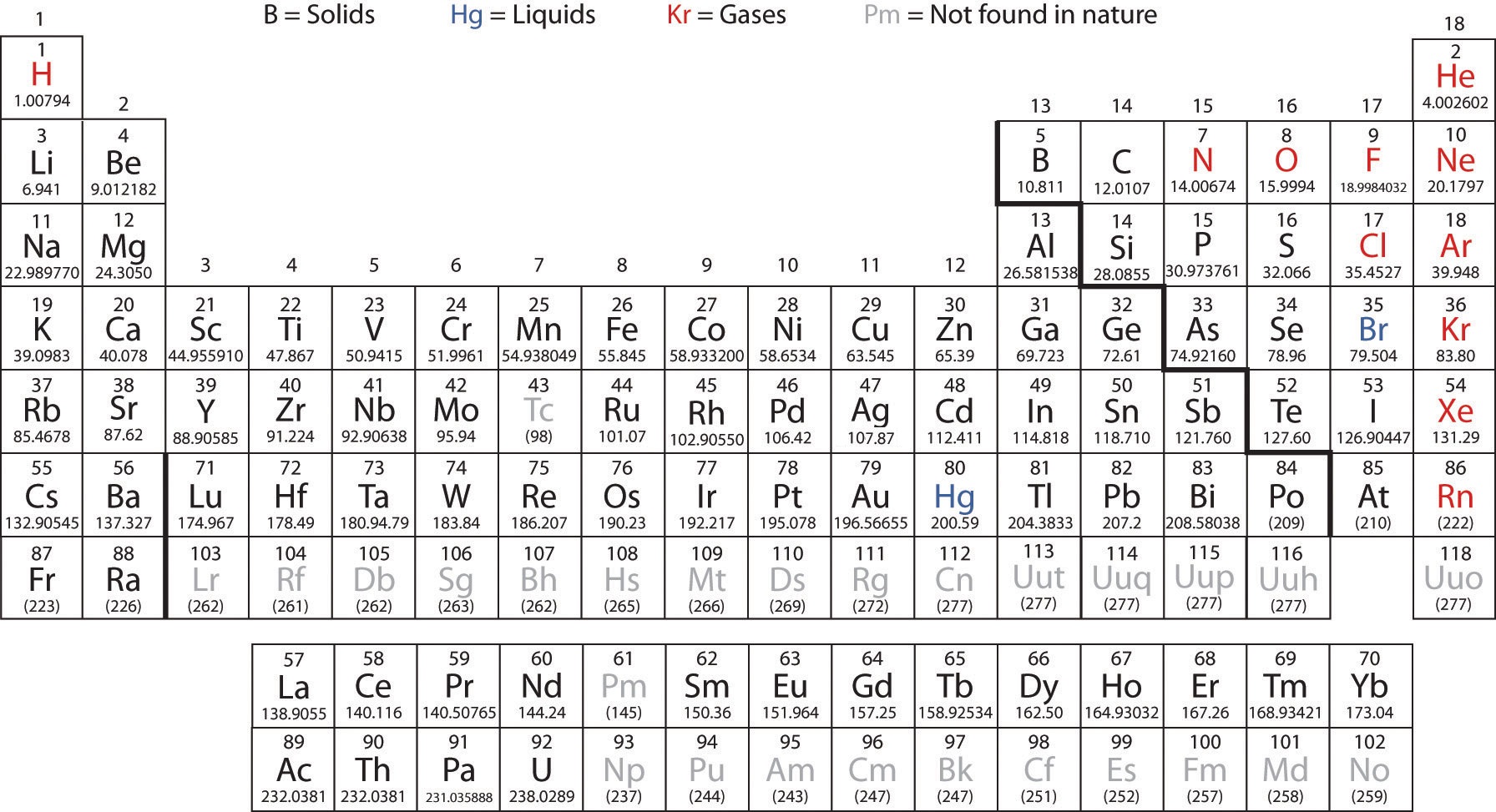
Periodic table mass units. An up to date periodic table with detailed but easy to understand information. This list contains the 118 elements of chemistry. Options for hiding the symbol or name of the elements provide a handy learning aid for memorizing the periodic table.
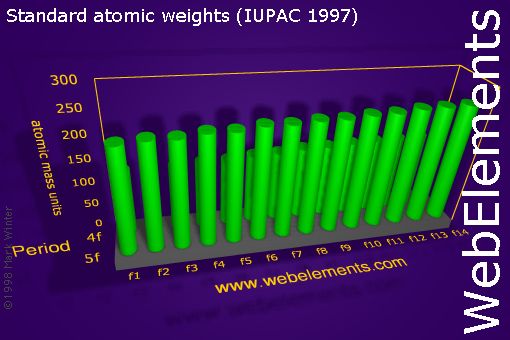
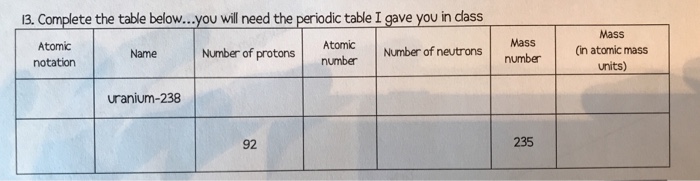
Atomic mass is measured in atomic mass units amu which are scaled relative to carbon 12 c that is taken as a standard element with an atomic mass of 12. The average number of neutrons for an element can be found by subtracting the number of protons atomic number from the atomic mass. Though individual atoms always have an integer number of atomic mass units the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element.
Click on any elements name for further information on chemical properties environmental data or health effects. It is the atomic weight or relative atomic mass that is listed in the box on the periodic table for each element. Thus each proton and neutron has a mass of about 1 amu.
The periodic table provides us with a comprehensive view of the various elements. The isotopes c13 and c14 will have a small effect on the average reported in the periodic table. This isotope of carbon has 6 protons and 6 neutrons.
Many elements have isotopes that will give cause the atomic mass to differ from the most common isotope. The unit mass for an atom element on the periodic table is not a whole number. Along with atomic mass and atomic number values this table helps us to understand the various properties abbreviations and names of all the elements present in nature.
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit amu also known as daltons dthe atomic mass is a weighted average of all of the isotopes of that element in which the mass of each isotope is multiplied by the abundance of that particular isotope. The following article will help you to gain more information about the same. Full descriptions from write up sources.
It is a decimal number due to the fact that there are different isotopes for each element and the atomic weight is a weighted average of all the isotopes for that element and their relative abundance. Ios app is also available. Use this periodic table for calculating molar mass for any chemical formula.

























0 Response to "Periodic Table Mass Units"
Post a Comment