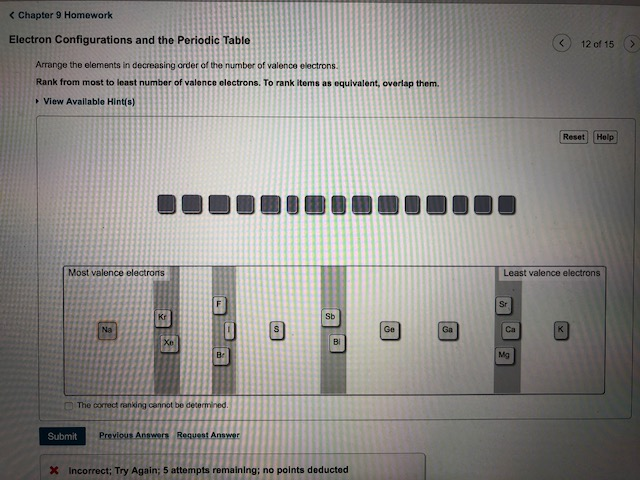
Periodic Table Of Elements With Number Of Valence Electrons
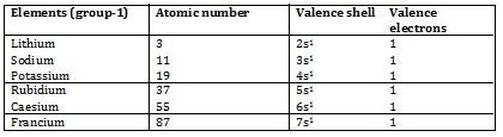
You may assume that the valences of the elementsthe number of electrons with which an atom will bond or formare those that can be derived by looking at the groups columns of the periodic table. In the periodic table the elements are listed in order of increasing atomic number z.
Valence electrons are the electrons present in the outermost shell of an atom.
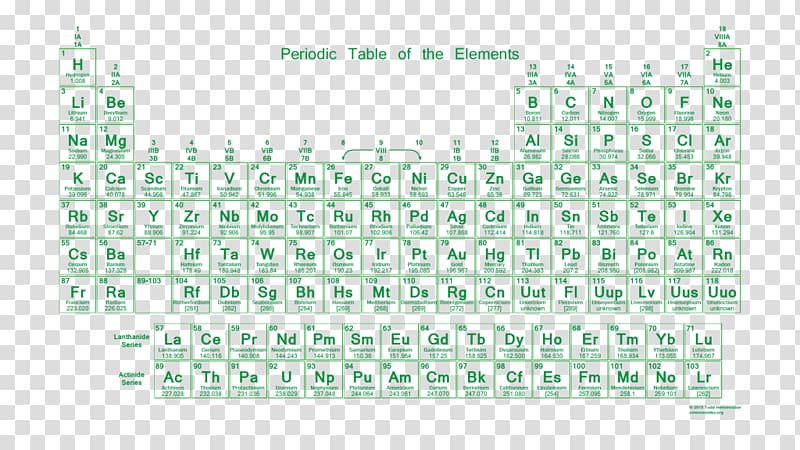
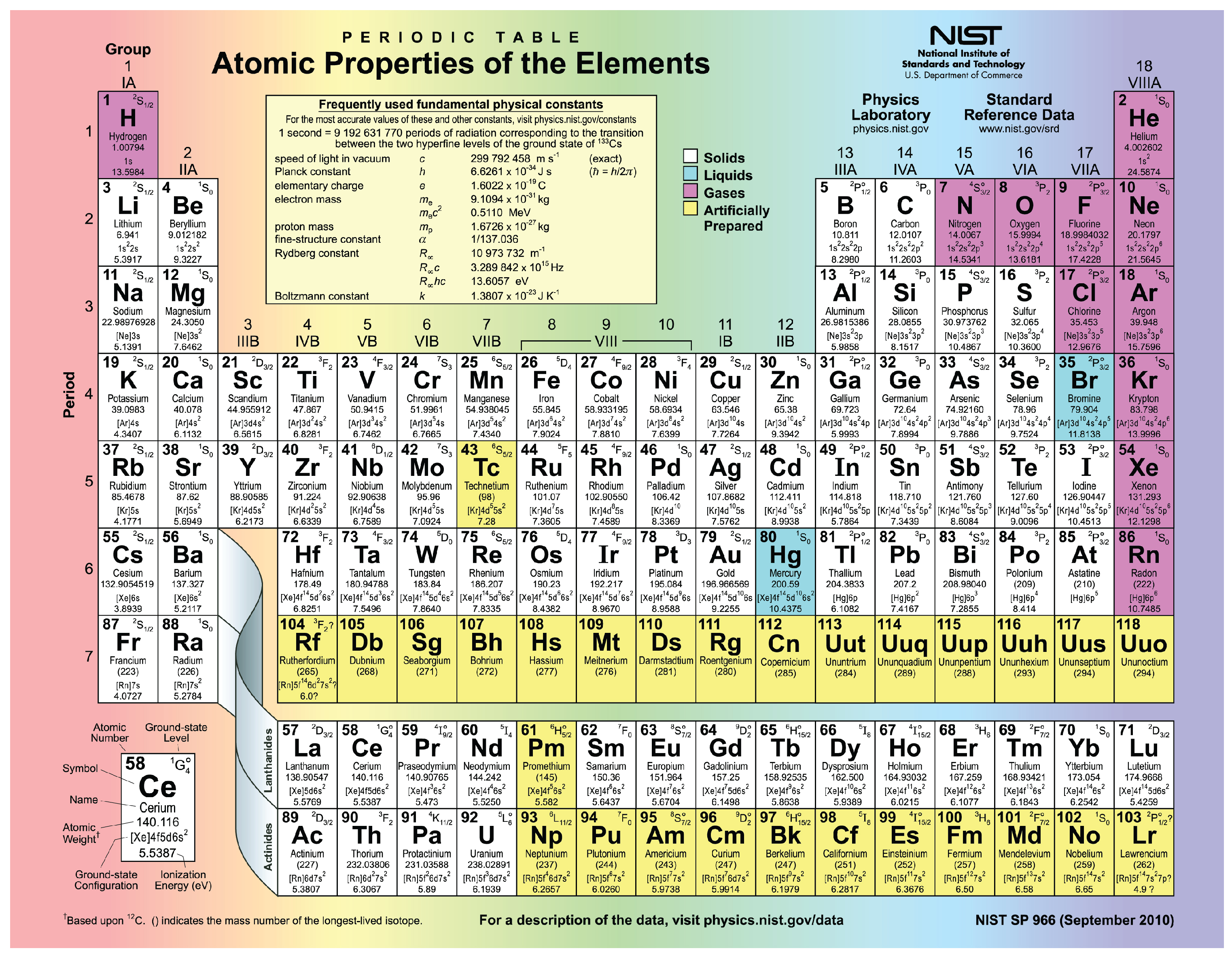
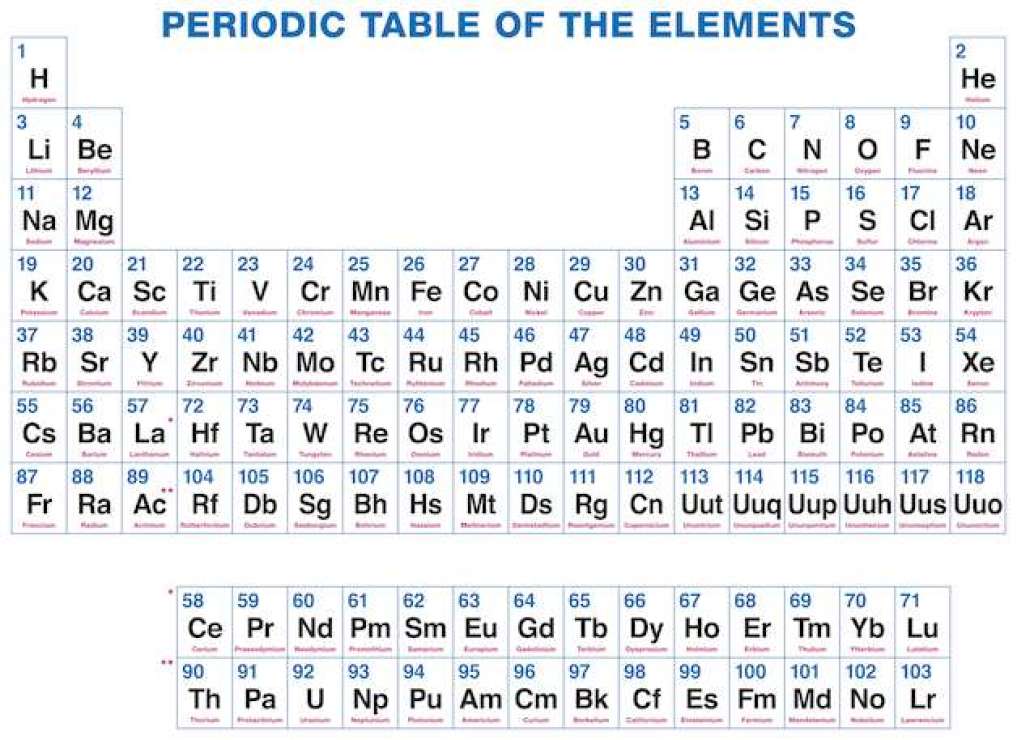
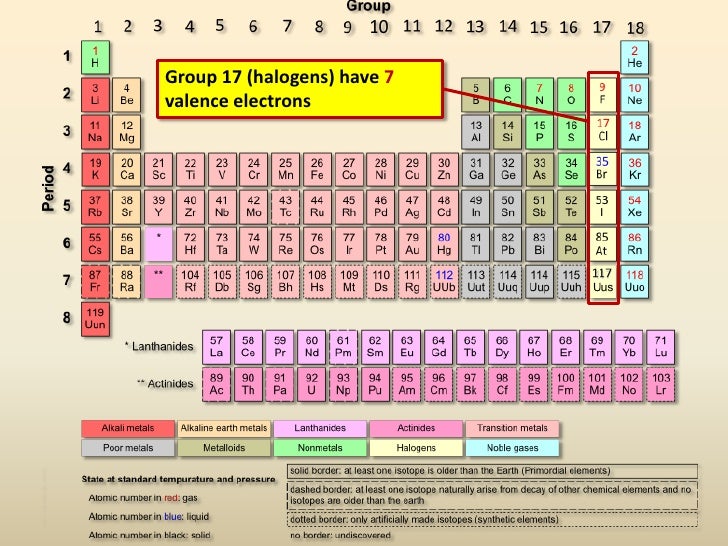
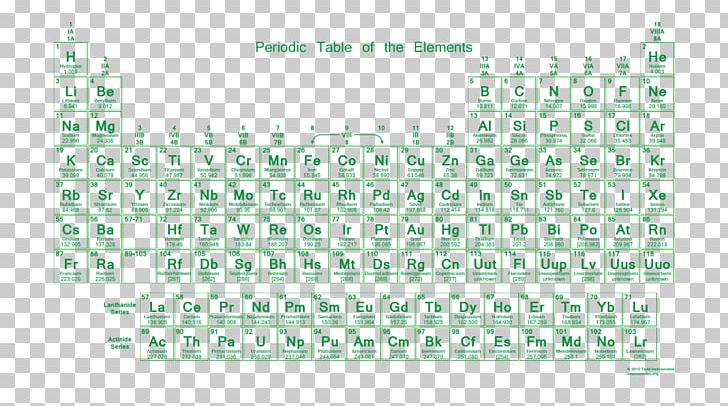
Periodic table of elements with number of valence electrons. A simpler version listing only the most common valence charges is also available. The vertical columns of the periodic table counting left to right 1 through 18 are called groups. The number of electrons in each elements electron shells particularly the outermost valence shell is the primary factor in determining its chemical bonding behavior.
Click on element atomic number element symbol element name and element valence electrons headers to sort. While these are the most common valences the real behavior of electrons is less simple. Periods correspond to the number of electron shells possessed by atoms of the elements in that row.
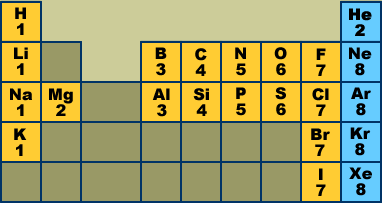
Full descriptions from write up sources. This information is available on a color periodic table of the elements or a black and white version. If your periodic table doesnt already have each column numbered give each a number starting with 1 for the far left end and 18 for the far right end.
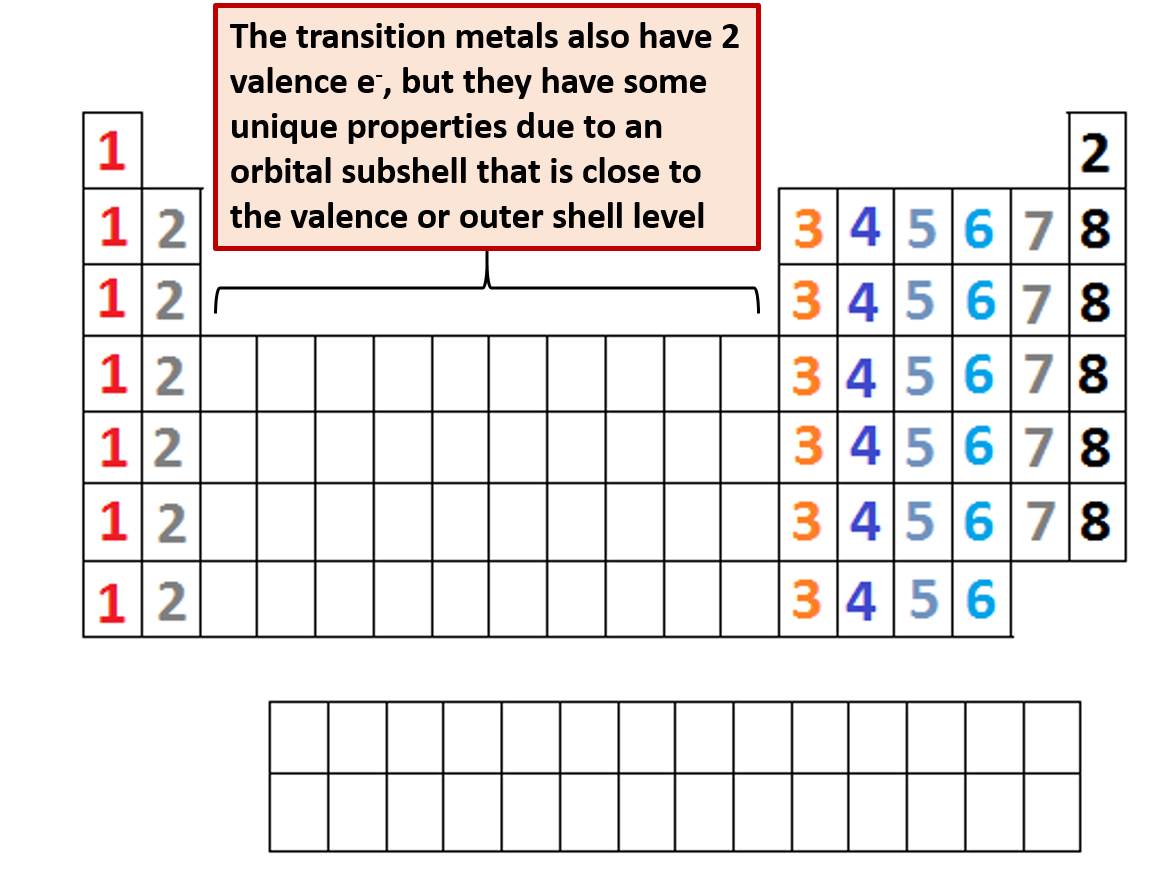
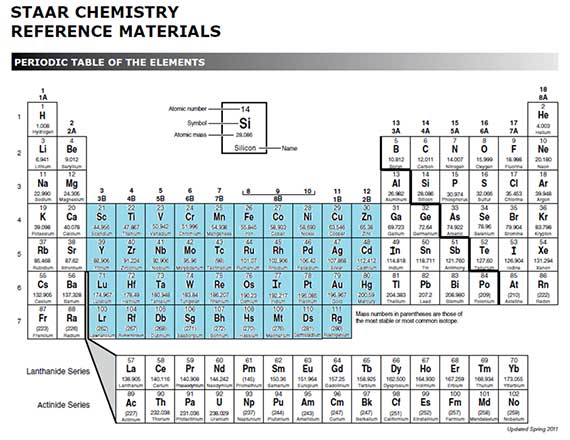
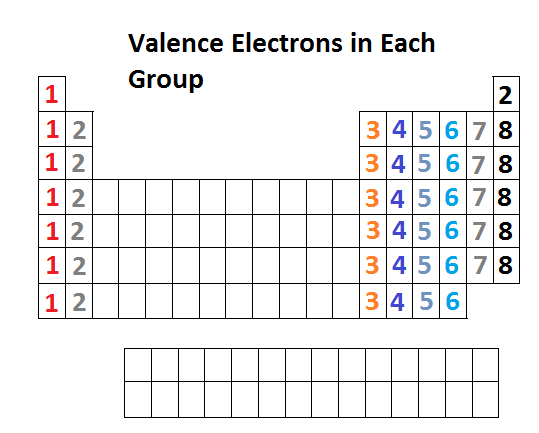
Label each column on the periodic table of elements from 1 to 18. Generally on a periodic table all of the elements in a single vertical column will have the same number of valence electrons. Todd helmenstine this table also contains the element number element symbol element name and atomic weights of each element.
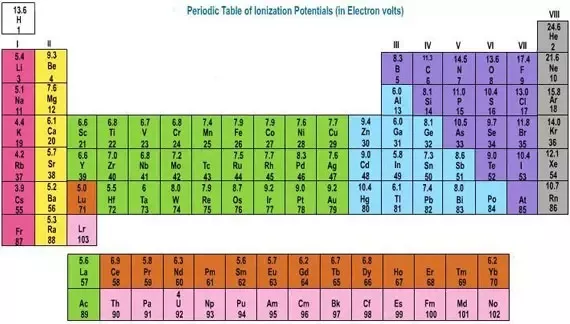
This valence electrons chart table gives the valence electrons of all the elements of periodic table. The two tables below show some examples of different compounds their valence diagrams and the valences for each element of the compound. Valence diagrams of a compound represent the connectivity of the elements with lines drawn between two elements sometimes called bonds representing a saturated valency for each element.
The configuration of these electrons follows from the principles of quantum mechanics. For example atoms in groups 1 and 2 have 1 and 2 valence electrons respectively. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search.
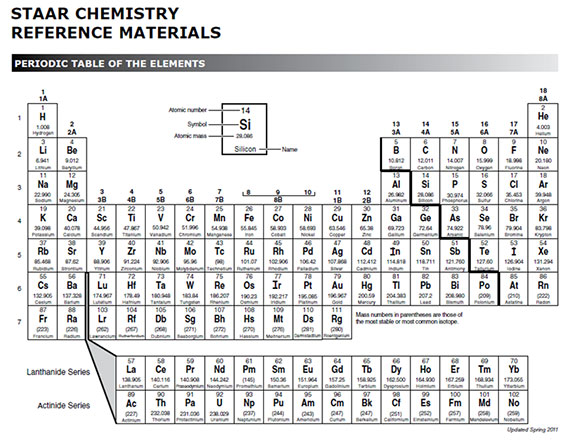
You can easily determine the number of valence electrons an atom can have by looking at its group in the periodic table. This is a table of the valences or oxidation states of the elements. The horizontal rows of the periodic table from 1 to 7 are called periods.
Atoms in groups 13 and 18 have 3 and 8 valence electrons respectively. This printable periodic table contains the atomic number element symbol element name atomic weights and most common valence charges. Determine the group number and period number of the element.
The most common valences are in boldvalues in italics are predicted theoretical values. In the periodic table elements with similar chemical properties are in the same group.












:max_bytes(150000):strip_icc()/PeriodicTableValence-58b5d8f95f9b586046df59fb.jpg)
/186810031-56a130cd5f9b58b7d0bce8ee.jpg)















0 Response to "Periodic Table Of Elements With Number Of Valence Electrons"
Post a Comment