Periodic Table Halogens Properties
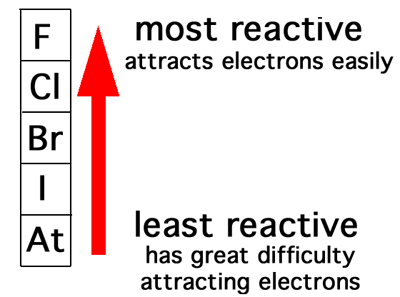
Although astatine is radioactive and only has short lived isotopes it behaves similar to iodine. Halogen any of the six nonmetallic elements that constitute group 17 group viia of the periodic table.
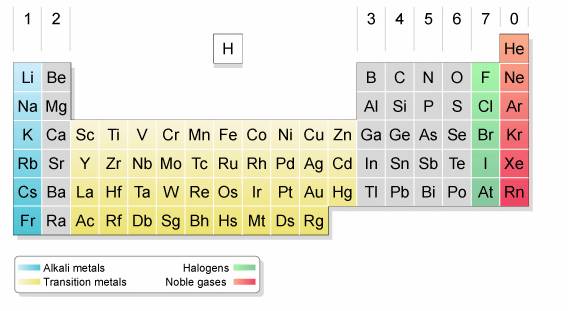
Group 7 is on the right hand side of the periodic table next to group 0.
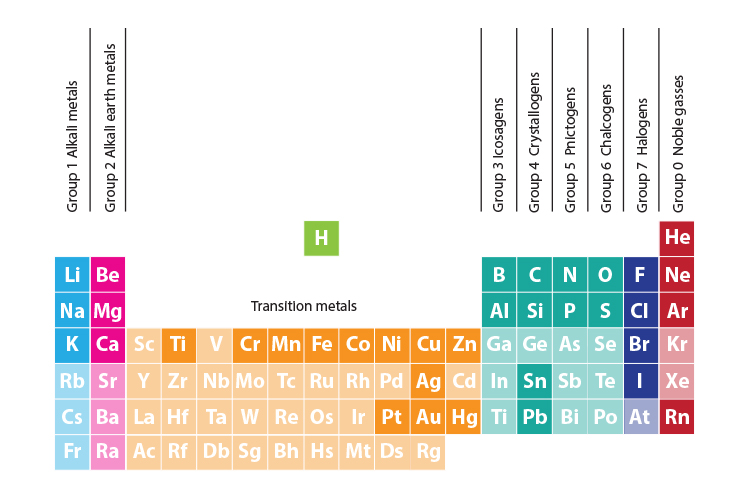
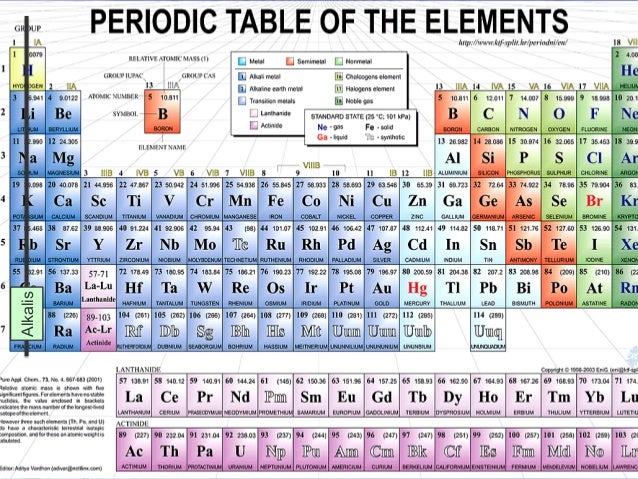
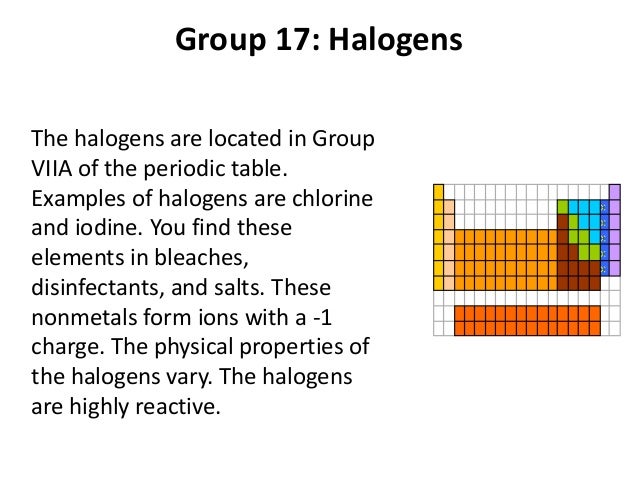
Periodic table halogens properties. The element group is a particular class of nonmetals. They can be found toward the right hand side of the table in a vertical line. The halogens are located in group viia of the periodic table or group 17 using iupac nomenclature.
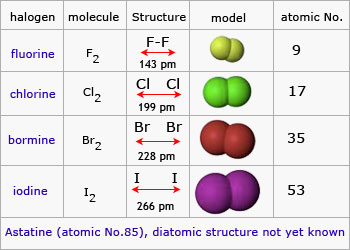
In this lesson you will learn about a unique group known as the halogen family. The halogen elements are fluorine f chlorine cl bromine br iodine i astatine at and tennessine ts. The halogens exist as simple.
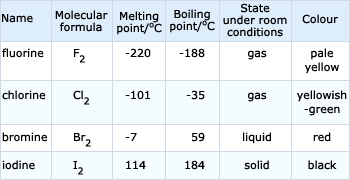
Ununseptium is artificially created element. The table shows the colour and. Down the group atom size increases.
Elements are grouped into families on the periodic table based on their chemical and physical properties. When halogens react with metals they produce a wide range of salts including calcium fluoride sodium chloride common table salt silver bromide and potassium iodide. The halogens are located on the right side of the modern periodic table before the inert gases the halogens are elements of group 7 a 17 in p block the halogens are mono valent elements as their outermost energy levels have 7 electrons.
These five toxic non metallic elements make up group 17 of the periodic table and consist of. The halogens show trends in their physical and chemical properties. The halogens show trends in their physical.
Group 7 is on the right hand side of the periodic table next to group 0. Learn more about the properties of halogens in this article. Fluorine f chlorine cl bromine br iodine i and astatine at.
All of the halogens. The halogens are located on the left of the noble gases on the periodic table. Halogens are nonmetals in group 17 or vii of the periodic table.
The term halogen means salt former and compounds containing halogens are called salts. The halogens are located in group 17 viia of the periodic table and belongs to a class of nonmetals. The halogen elements are fluorine chlorine bromine iodine astatine and ununseptium.
The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. As a diatomic molecule fluorine has the weakest bond due to repulsion between electrons of the small atoms.

/periodic-table-935378844-5c52765bc9e77c0001380acb.jpg)













/complete-periodic-table-of-elements-royalty-free-vector-166052665-5a565f0e47c2660037ab8aca.jpg)











0 Response to "Periodic Table Halogens Properties"
Post a Comment