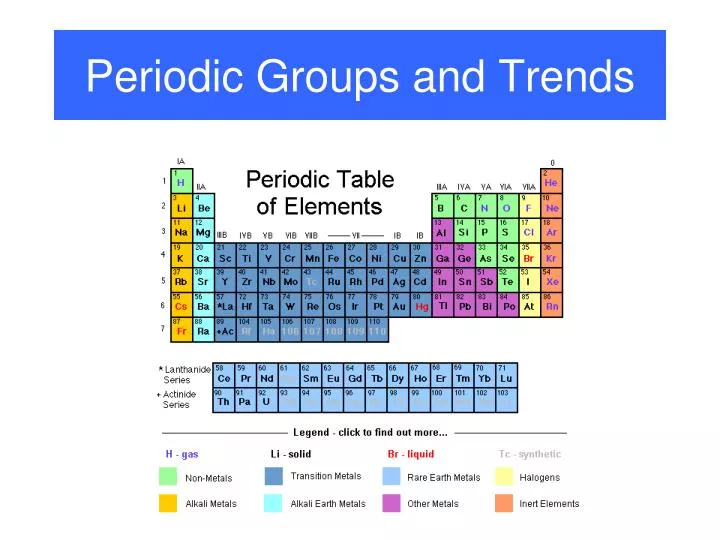
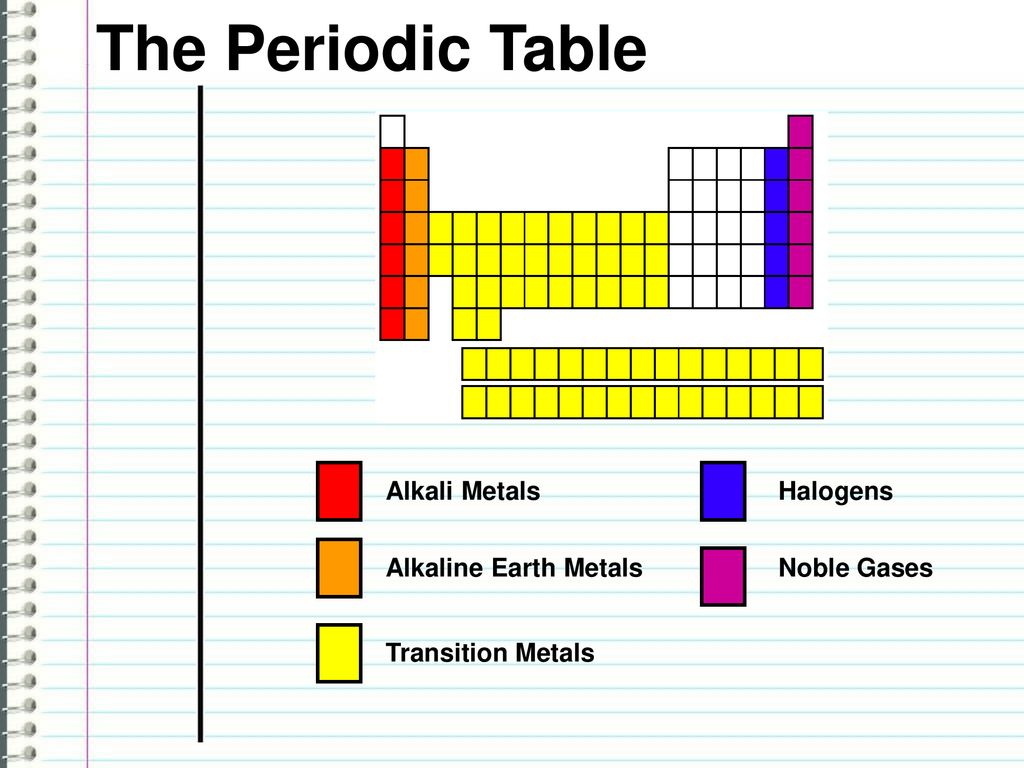
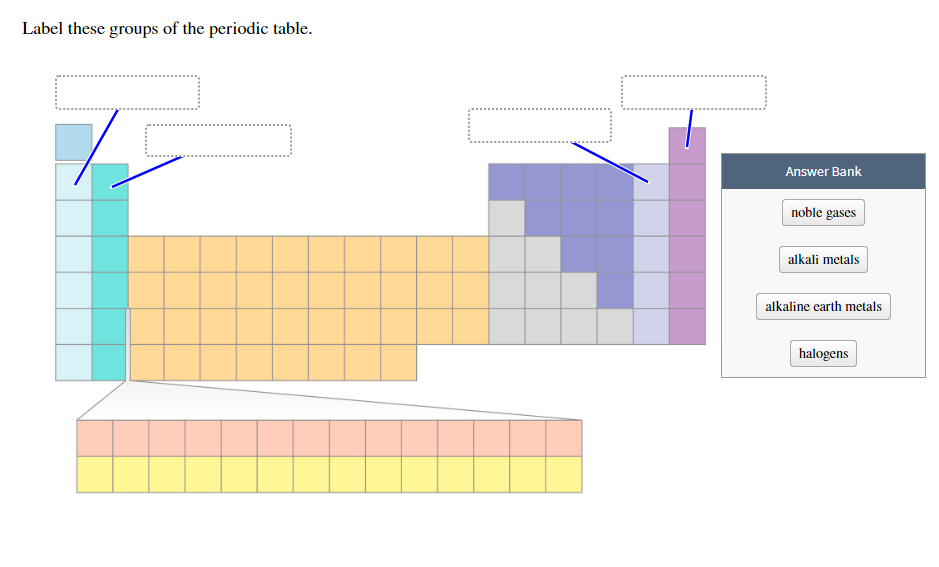
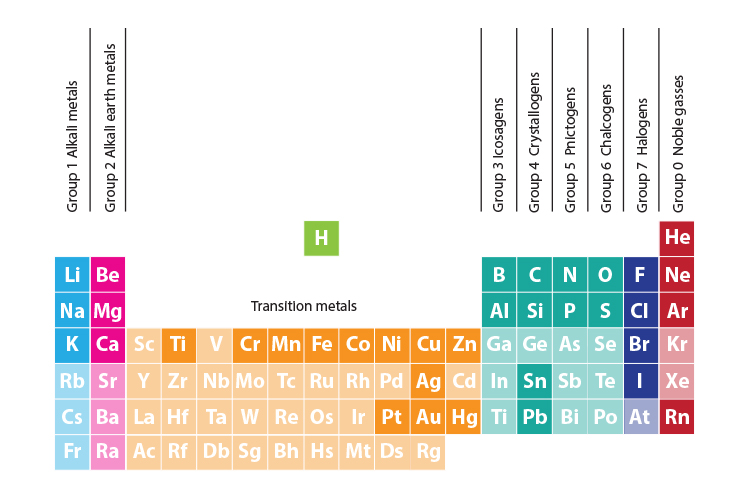
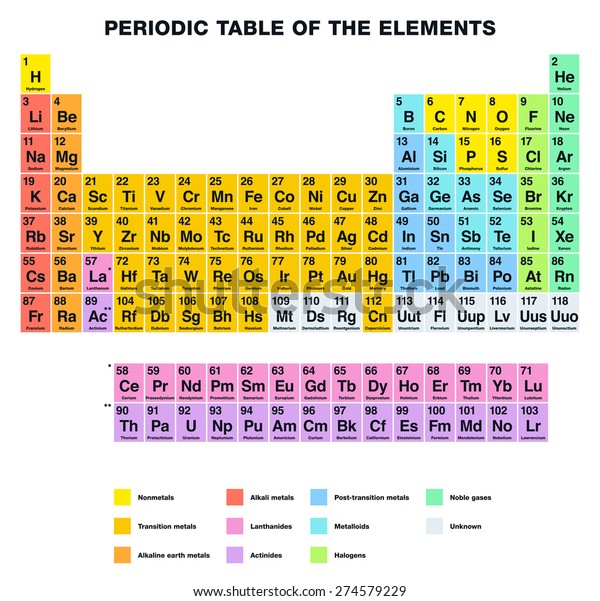
Periodic Table Halogens Alkali Earth Metals
The alkali metals are among the most electropositive elements on the periodic table and thus tend to bond ionically to the most electronegative elements on the periodic table the halogens fluorine chlorine bromine iodine and astatine forming salts known as the alkali metal halides. Here is a look at the location and the properties of these elements.
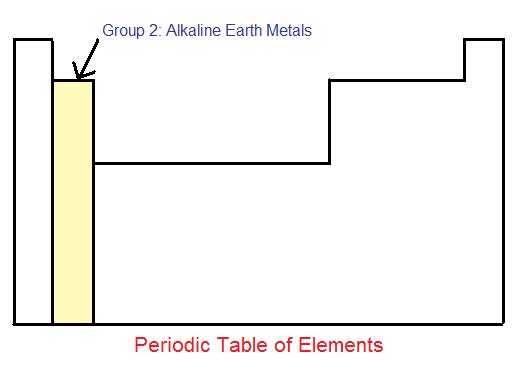
The alkaline earth metals are one group of elements on the periodic table.

Periodic table halogens alkali earth metals. Periodic table of the elements groups alkali metals alkaline earth metals blocks gases stp halogens lanthanidesactinides liquids stp main group metalloids metals noble gases non metals solids stp transition metals. The elements highlighted in yellow on the periodic table in the graphic belong to the alkaline earth element group. Group 17 is known as the.
Solid liquid and gas. You will find the alkaline earth metals right next door in group ii. They are ready to give up those two electrons in electrovalent ionic bonds.
In chemical terms all of the alkaline earth metals react with the halogens to form the alkaline earth metal halides all of which are ionic crystalline compounds except for beryllium chloride which is covalent. Group 2 is known as the alkaline earth metals and as predicted are less reactive than group 1. Each alkaline earth metal atom has two valence electrons in its outermost shell s orbital.
Well keep working our way in from the edges of the periodic table. Heading to group two so we just covered the alkali metals in group i. The chemical reaction of the alkali metals with the halogens is.
This gives alkaline earth metals the largest atomic radii in their periods. The reaction is very vigorous and can sometimes result in explosions. It is the only element group that includes elements capable of existing in three of the four main states of matter at room temperature.
This group lies in the s block of the periodic table. This is the second most reactive family of elements in the periodic tabledo you know why they are called alkalinewhen these compounds are mixed in solutions they are likely to form solutions with a ph greater than 7. 2 m x 2 2 mx where m represents an alkali metal and x represents a halogen when alkali metals react with nitogen nitrides are formed.
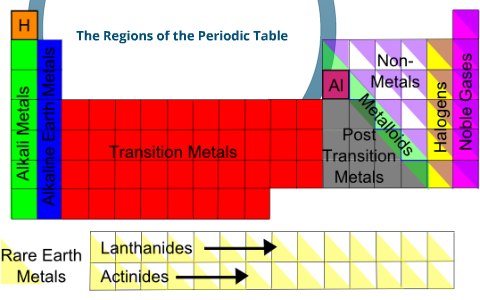
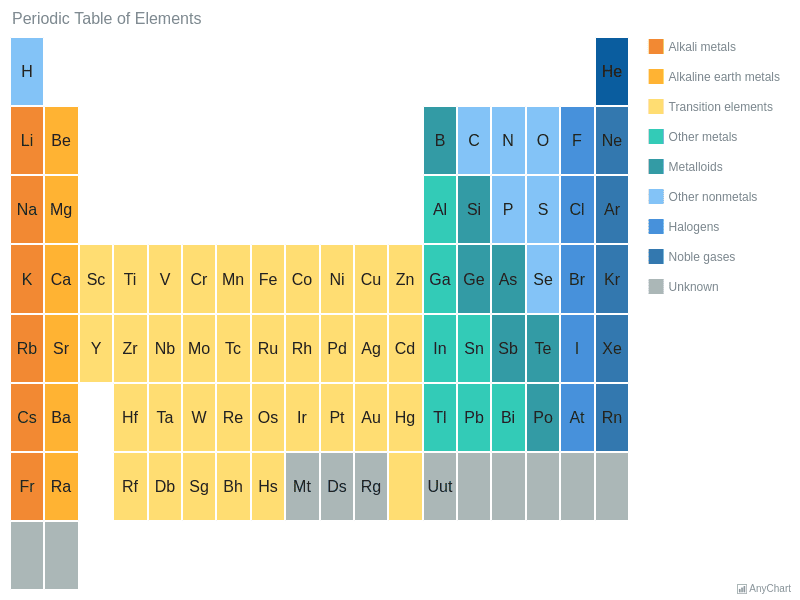
In our version of the table we have chosen the most commonly accepted demarcations between these elements. The alkaline earth metals are all silver colored and soft and have relatively low densities melting points and boiling points. Periodic table or periodic chart of elements showing alkaline earth metals.
For example in some tables group 12 is is categorized with the post transition metals and in others aluminum and tin are included characterized as metalloids or poor metals. The halogens are a group of elements on the periodic table.

/complete-periodic-table-of-elements-royalty-free-vector-166052665-5a565f0e47c2660037ab8aca.jpg)




















0 Response to "Periodic Table Halogens Alkali Earth Metals"
Post a Comment