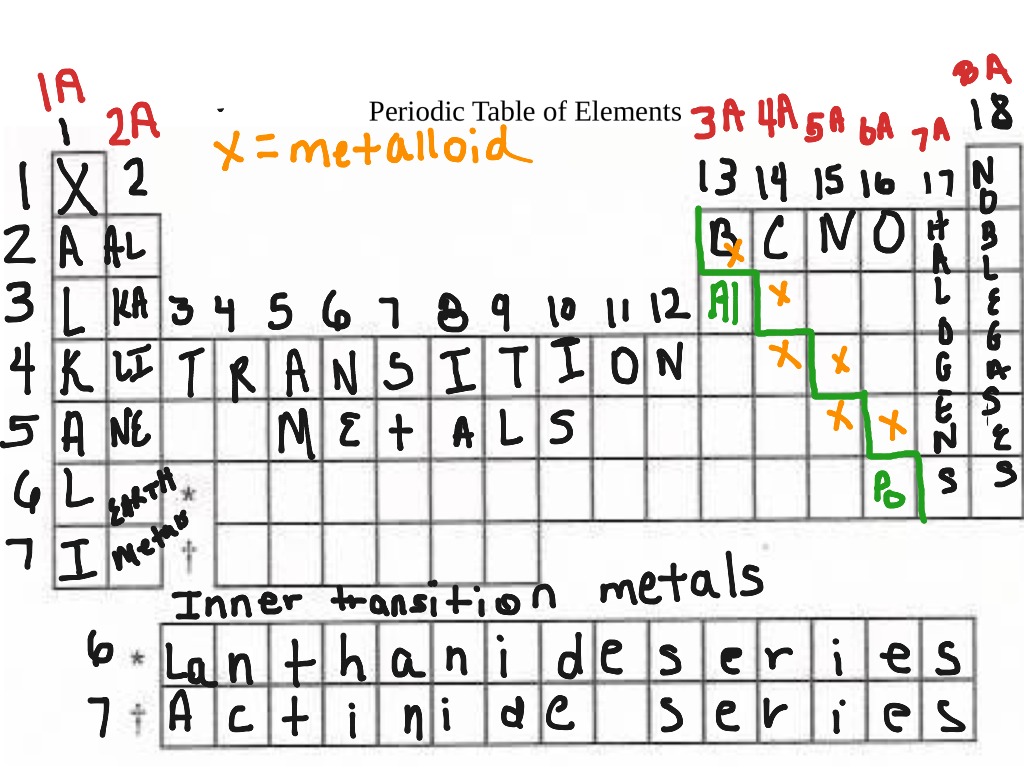
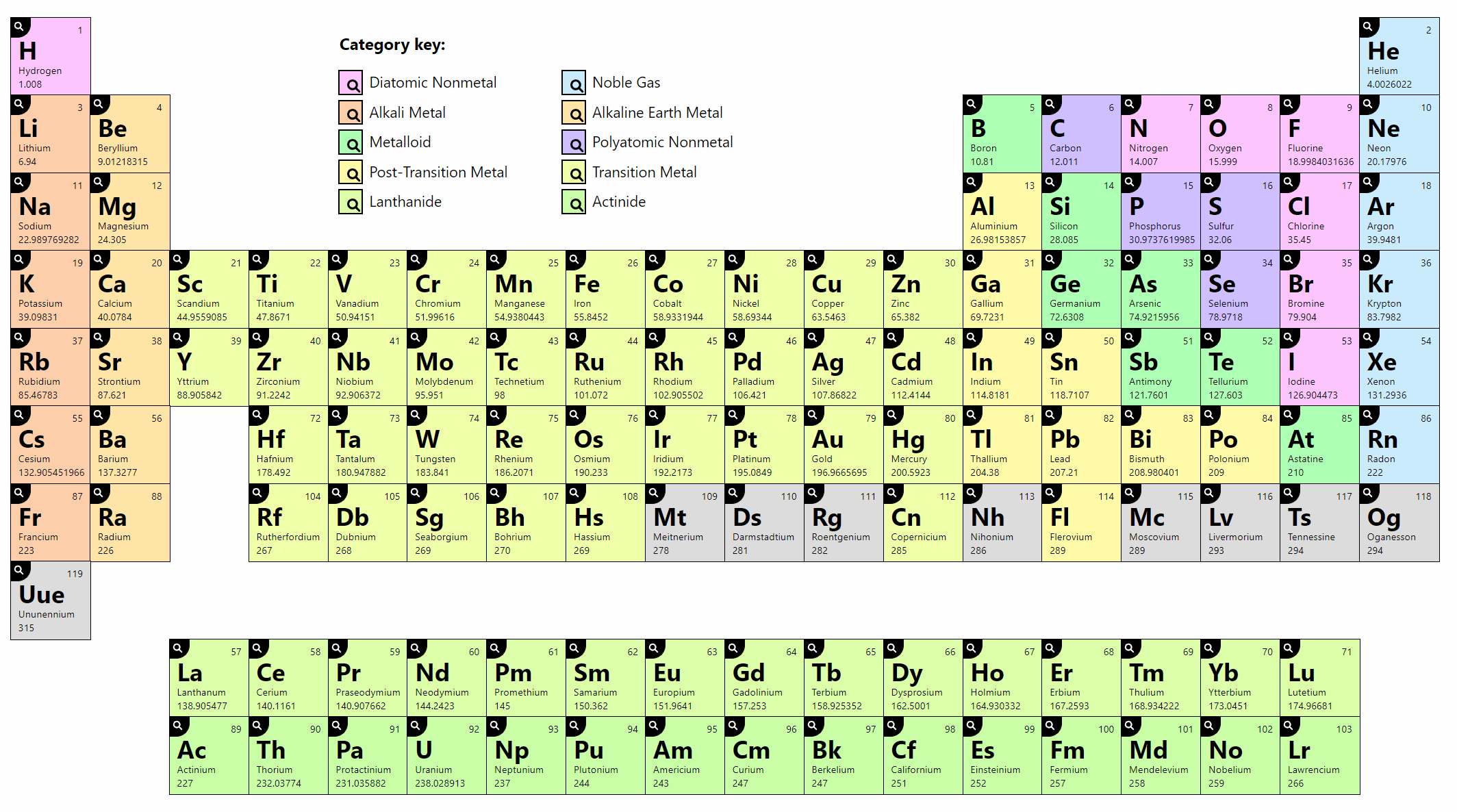
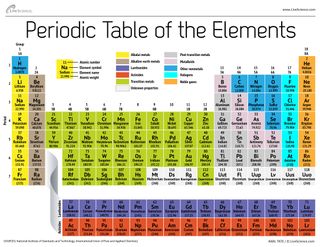
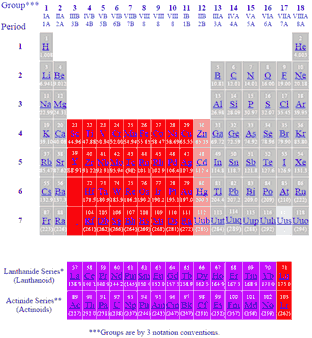
Labeled Inner Transition Metals Periodic Table
The lanthanides are very similar. A very well known group in the periodic table is that of inner transition metals.
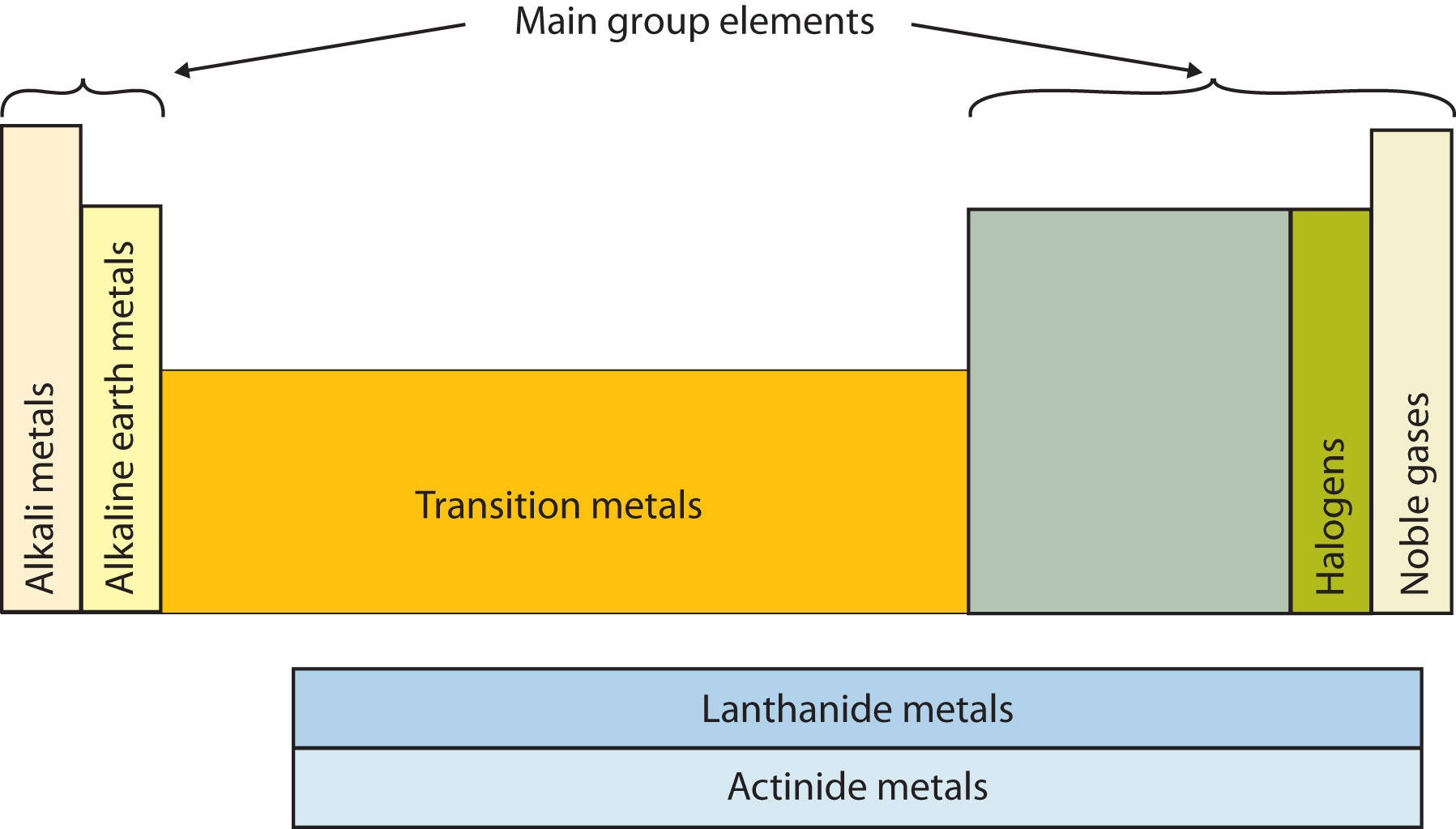
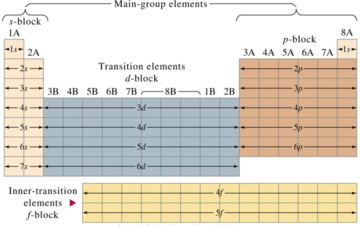
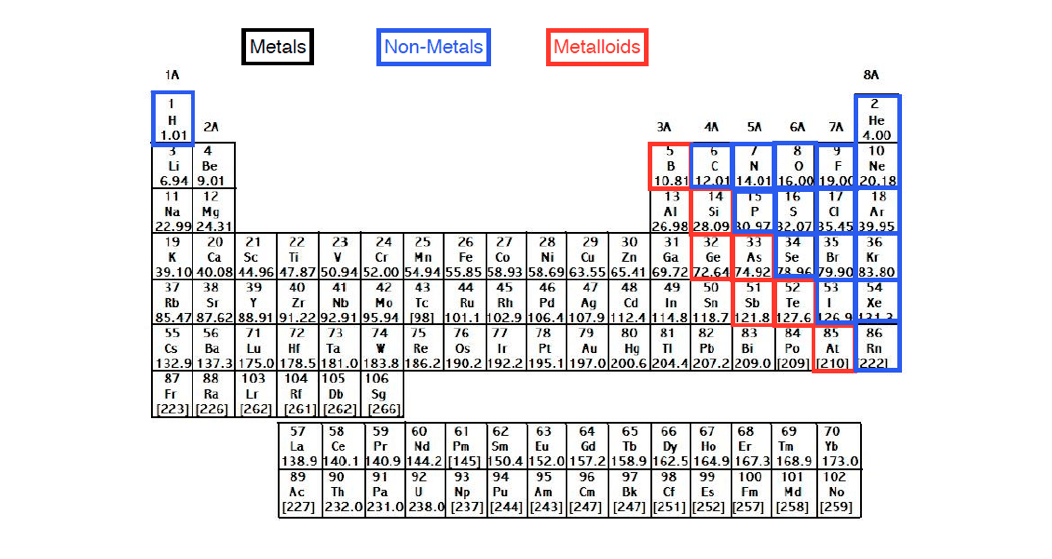
The p block is on the right side of the standard periodic table and encompasses elements in groups 13 to 18.
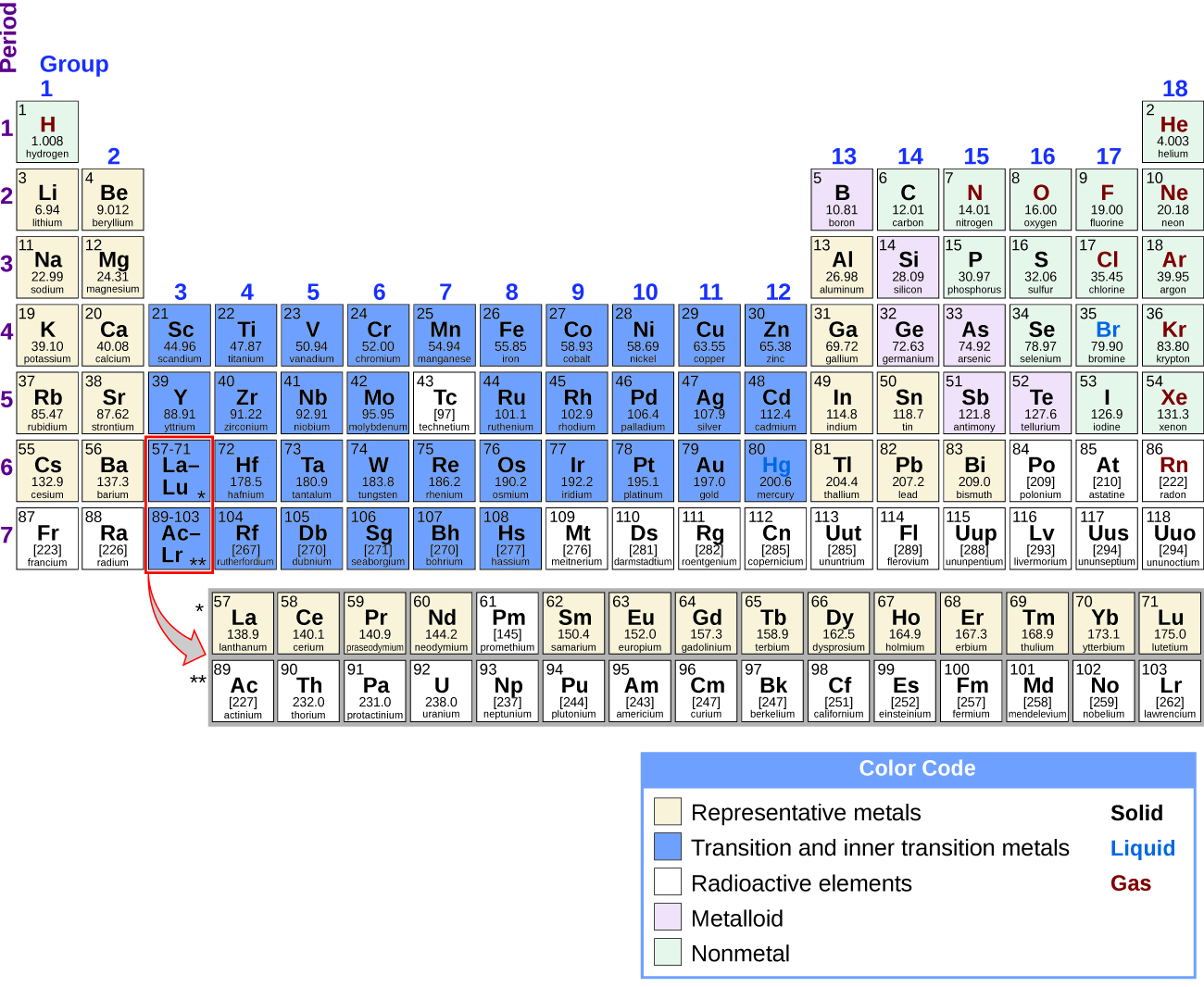
Labeled inner transition metals periodic table. An inner transition metal itm of chemical elements on the periodic tablethey are normally shown in two rows below all the other elements. Periodic table the inner transition metals are shown in two rows at bottom pink and purple 18 1 periodicity inner transition metals american naming system of 8 the completely labeled and colored periodic table the completely labeled and colored periodic table. Periodic table the inner transition metals are shown in two rows at bottom pink and purple 18 1 periodicity inner transition metals american naming system of 8 the completely labeled and colored periodic table the completely labeled and colored periodic tablepics of.
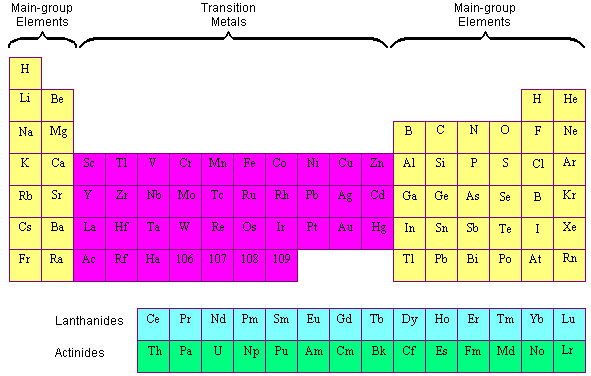
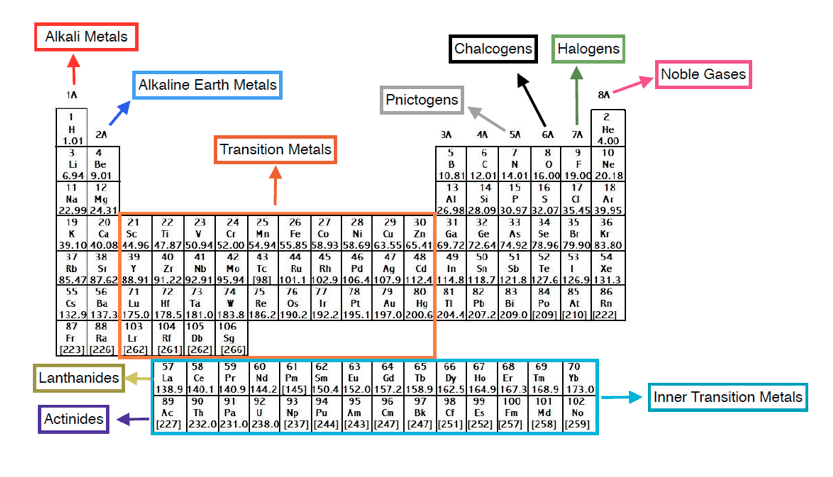
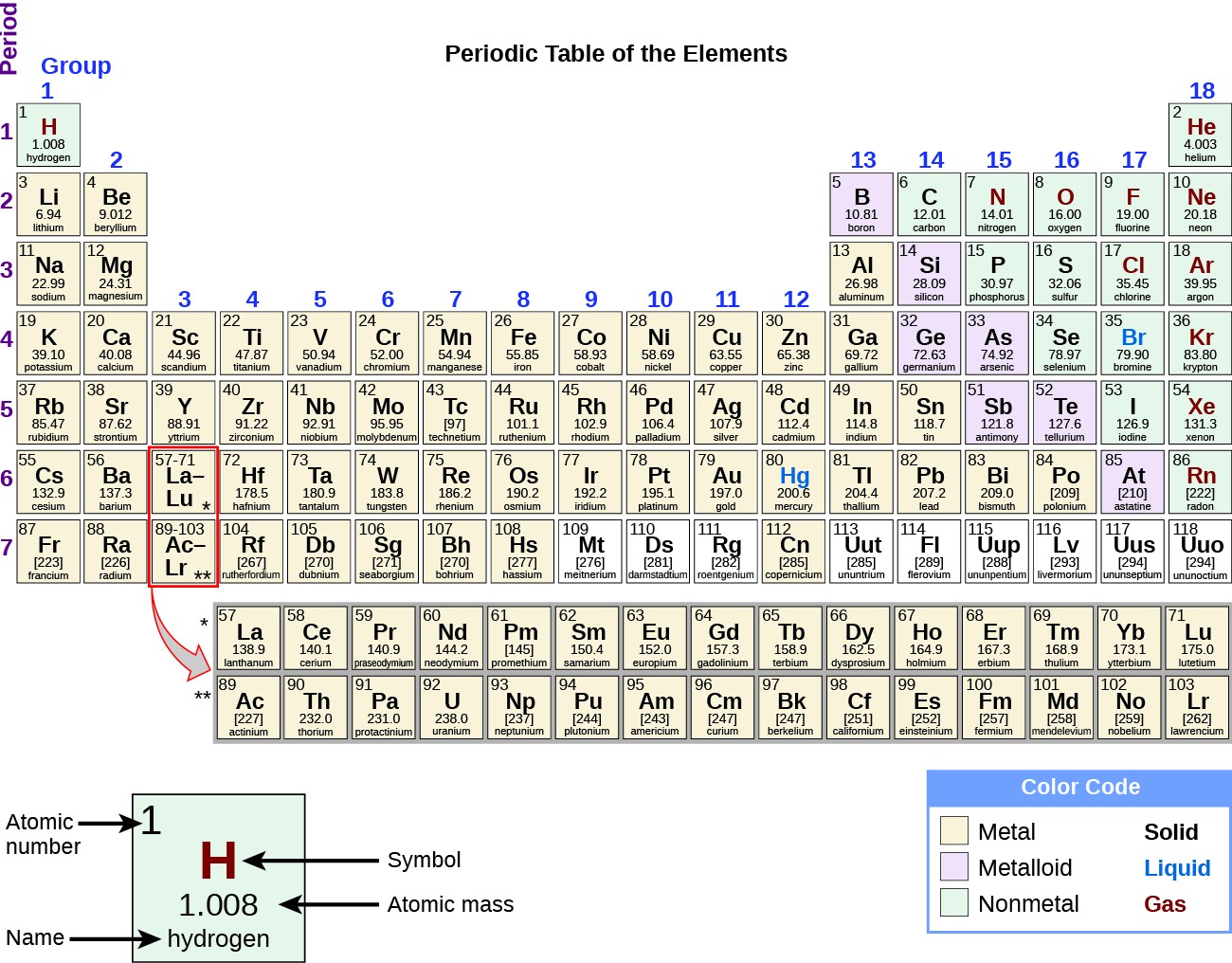
The name transition metal refers to the position in the periodic table of elements. Periodic table labeled inner transition metals source. You need to remember that those electrons are added to the second to last shells.
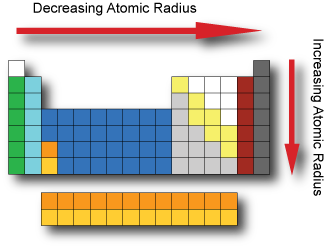
This is the point in the periodic table where you can place more than 8 electrons in a shell. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search. The transition metals or transition elements traditionally occupy all of the d block of the periodic table.
Helium though being the first element in group 18 is not included in the p block. Think about argon ar. The transition elements represent the successive addition of electrons to the d atomic orbitals of the atoms.
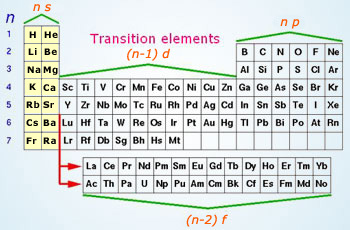
They include elements 57 71 known as lanthanides and 89 103 actinides. Itms have three incomplete outermost electron shells and are all metals. They include elements 57 71 or lanthanides and 89 103 or actinidesthe lanthanides are very similar and the actinides are all radioactive.
These elements were sometimes called rare earth elements or rare earth metals due to their extremely low natural occurrence. Lets find out the names and properties of these metals through this sciencestruck article. Lanthanides are located inperiod 6.
Their general electronic configuration is ns 2 np 16. Transition metals are able to put more than eight electrons in the shell that is one in from the outermost shell. It has 18 electrons set up in a 2 8 8 order.
Periodic table labeled inner transition metals. In this way the transition metals represent the transition between group 2 2a elements and group 13 3a elements. The inner transition elements occupy a position in between the elements lanthanum z57 and hafnium z72 and between actinium z89 and rutherfordium z104.
Metals of the s block form ionic compounds with the halogen nonmetals in group 17. This is where it starts. Scandium sc is only 3 spots away with 21 electrons but it has a configuration of 2 8 9 2.
Actinides are located inperiod 7. Full descriptions from write up sources. Elements 58 71 which follow lanthanum are the lanthanides and elements 90 103 which follow actinium are the actinides.
The actinides are all radioactive. Inner transition metals are usually put at the bottom of the periodic table.




















/ecblocks-56a129535f9b58b7d0bc9f2e.jpg)


0 Response to "Labeled Inner Transition Metals Periodic Table"
Post a Comment