Periodic Table Of Elements Metals And Nonmetals
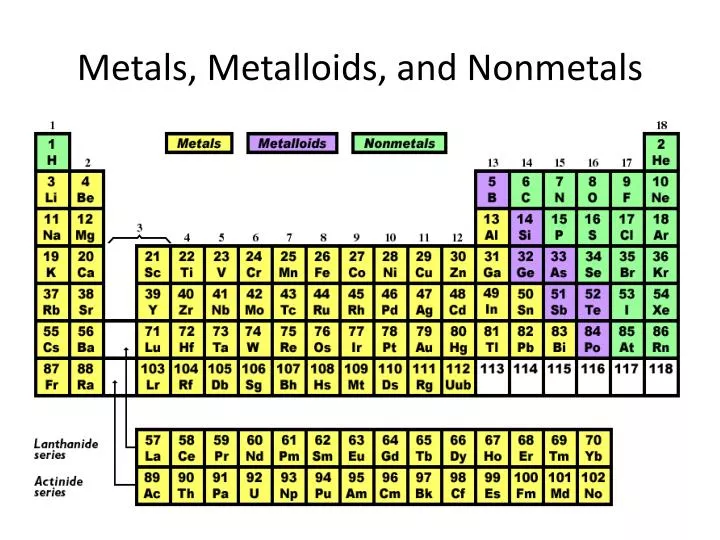
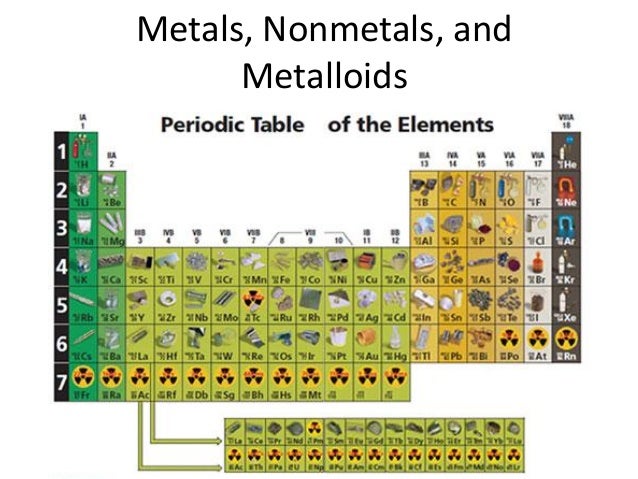
Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals. Metals metalloids and nonmetals 1 this entry was posted on august 27 2014 by todd helmenstine updated on january 29 2019 the elements of the periodic table can be broken into three different groups.
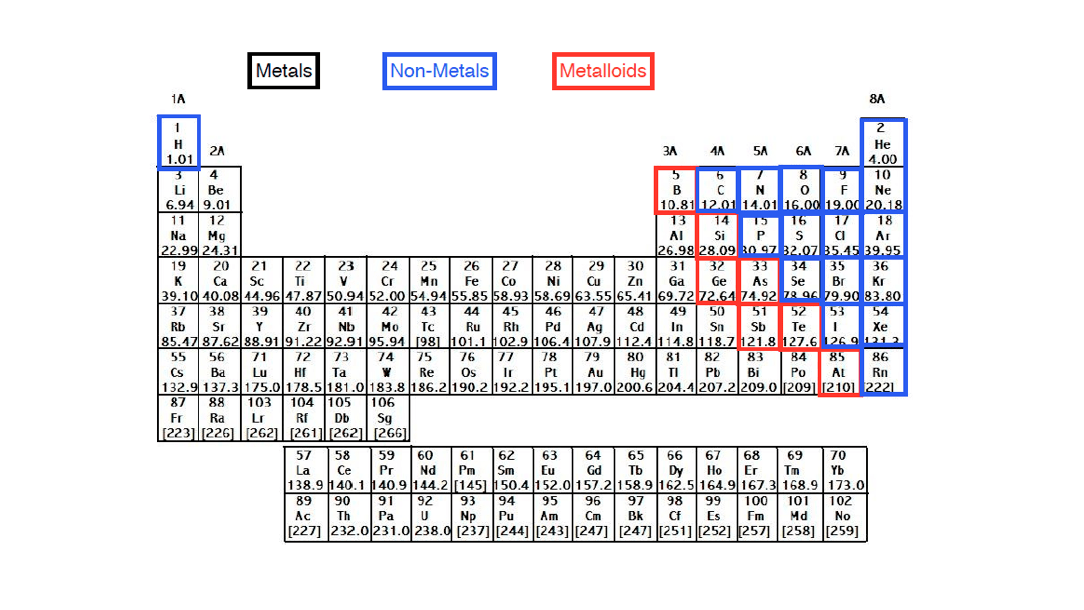
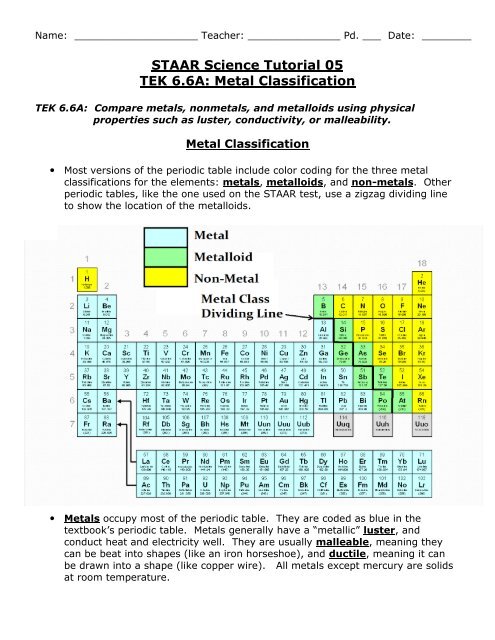
Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals.

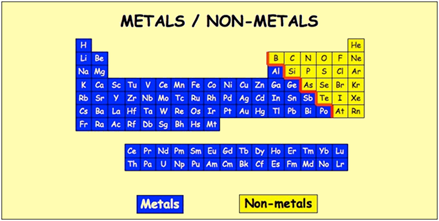
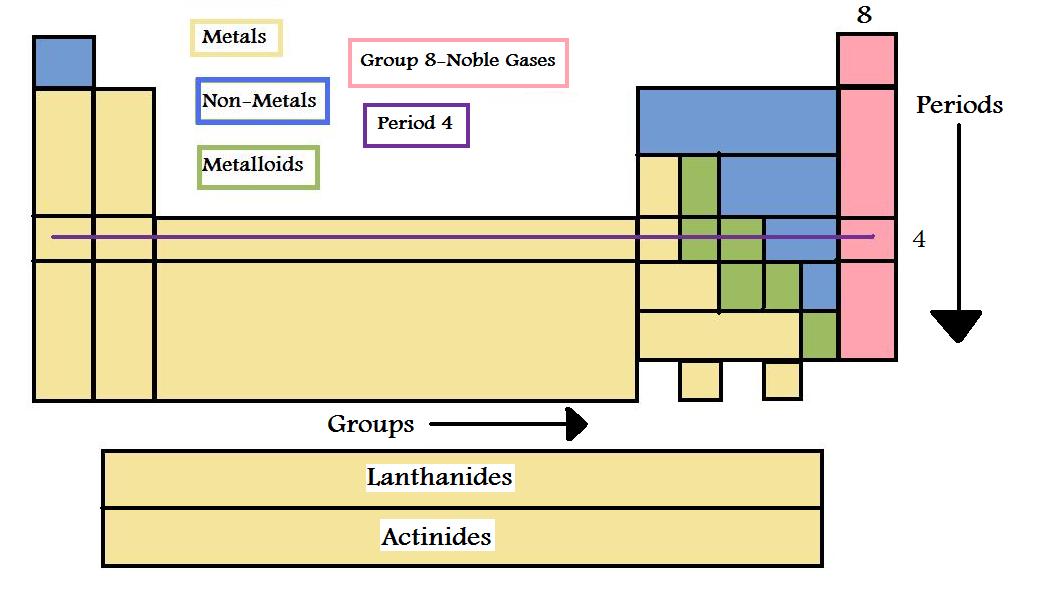
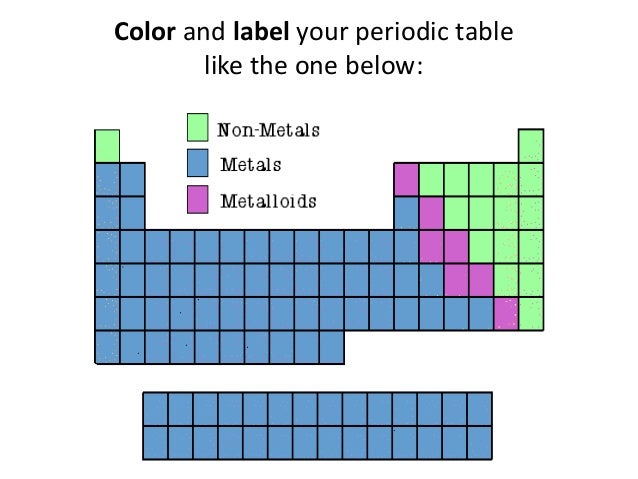
Periodic table of elements metals and nonmetals. The elements are divided into two main types metals and nonmetals based on their properties. Metals comprise the large majority of the elements and can be subdivided into several different categories. Metals metalloids and nonmetals.
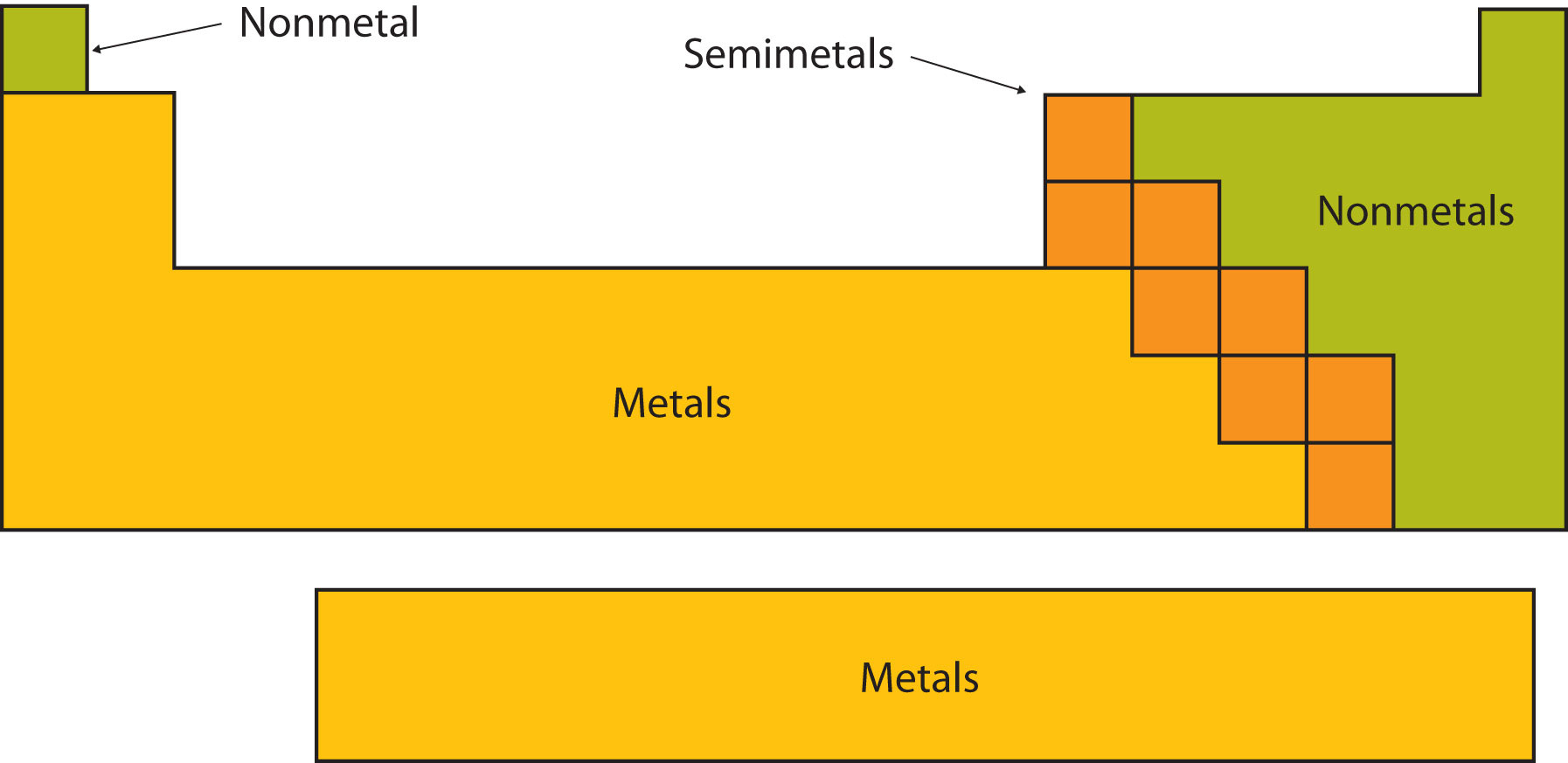
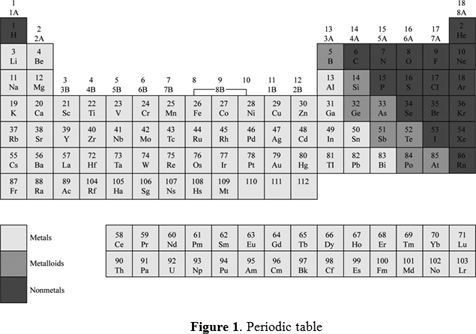
The metalloids separate the metals and nonmetals on a periodic table. In the periodic table you can see a stair stepped line starting at boron b atomic number 5 and going all the way down to polonium po atomic number 84. The nonmetals or non metals are a group of elements located on the right side of the periodic table except for hydrogen which is on the top left.
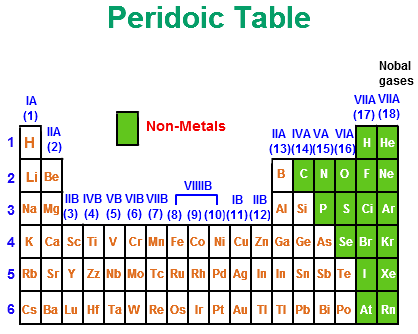
The non metals or non metallic elements. Hydrogen h carbon c nitrogen n oxygen o phosphorus p sulphur sulfer s selenium se uuo may belong here and the noble gases form a relatively small group with a step like pattern towards the left hand side of the periodic table hydrogen being the odd one out on the right of the. The periodic table contains a lot of useful information on the elements.
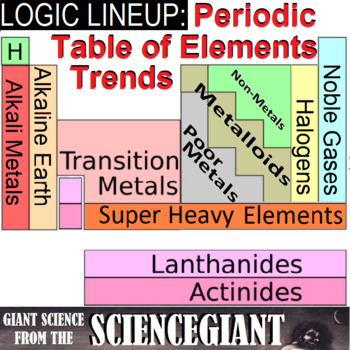
The less reactive alkaline earth metals lanthanides and radioactive actinides. When we study the elements it is important to know which elements are metals and. Using it you should be able to classify all the elements in different ways.
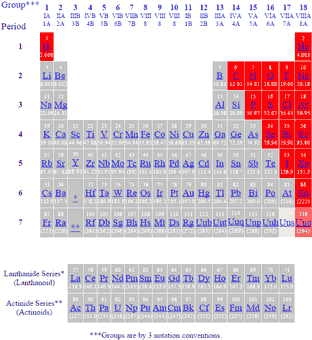
The periodic table of metals and nonmetals can be broken down to give you a sense of each elements characteristics. Only eighteen elements in the periodic table are generally considered asnonmetals. From left to right in the periodic table these categories include the highly reactive alkali metals.
The archetypal transition metals and the physically and chemically weak post transition metals. These elements are distinctive in that they typically have low melting and boiling points dont conduct heat or electricity very well and tend to have high ionization energies and electronegativity values. Except for germanium ge and antimony sb all the elements to the left of that line can be classified as metals.
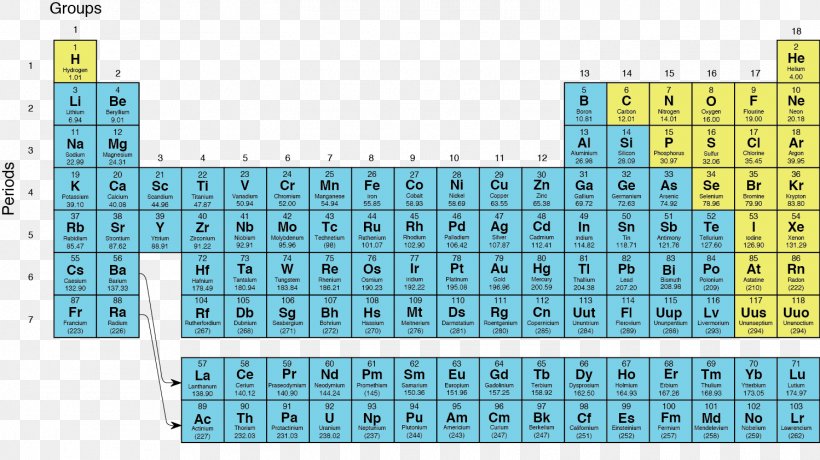
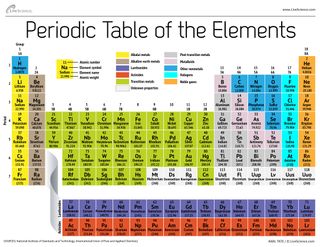
Elements to the left of the line are considered metals. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesthe structure of the table shows periodic trendsthe seven rows of the table called periods generally have metals on the left and nonmetals on the right. But nonmetals make up most of the crust atmosphere and oceans of the earth.
The nonmetals list which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. The nonmetals are located on the upper right side of the periodic table. Also many periodic tables have a stair step line on the table identifying the element groups.
The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. The line begins at boron b and extends down to polonium po.







/periodic-table--illustration-738787291-59888b4822fa3a00109a466d.jpg)









/Periodic-Table-Metals-56a12db33df78cf772682c44.png)
:max_bytes(150000):strip_icc()/nonmetals-56a12d975f9b58b7d0bccfd3.png)
:max_bytes(150000):strip_icc()/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)

0 Response to "Periodic Table Of Elements Metals And Nonmetals"
Post a Comment