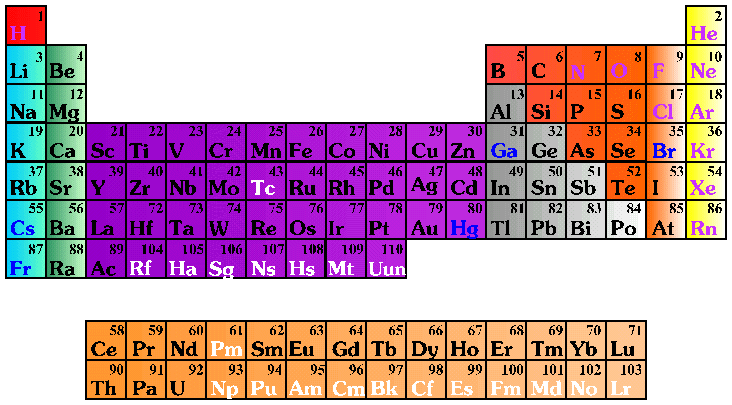
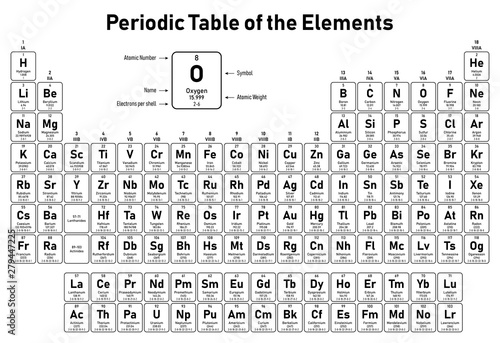
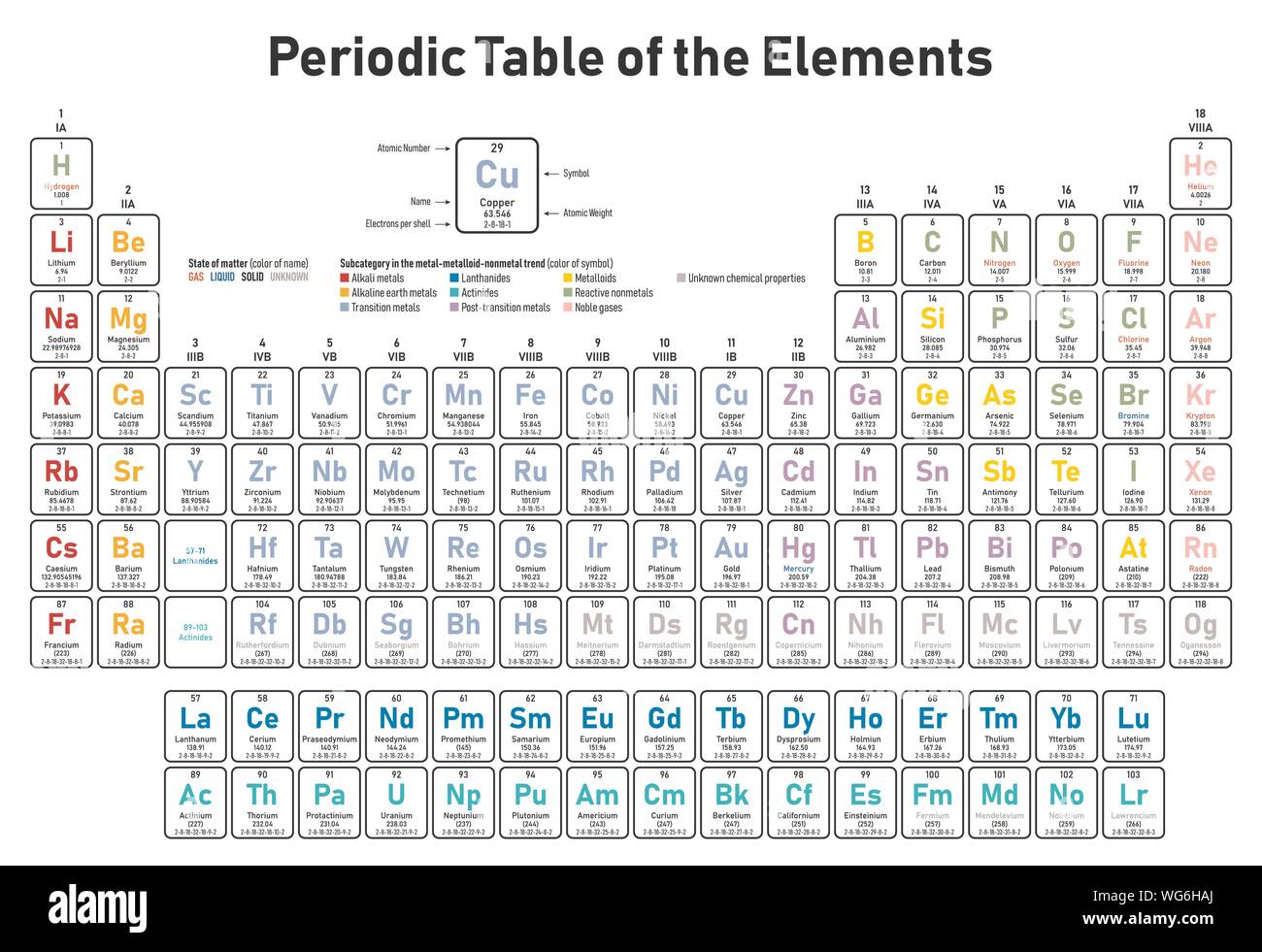
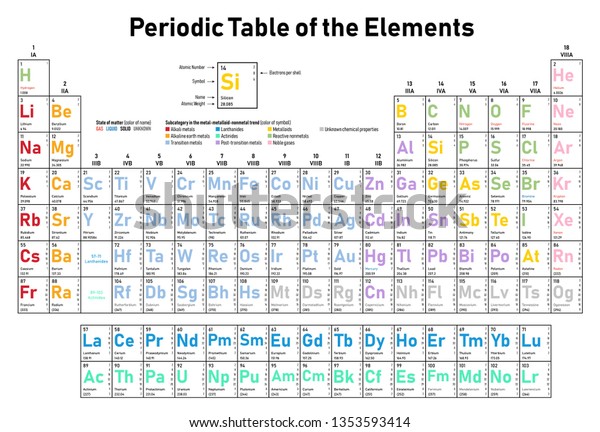
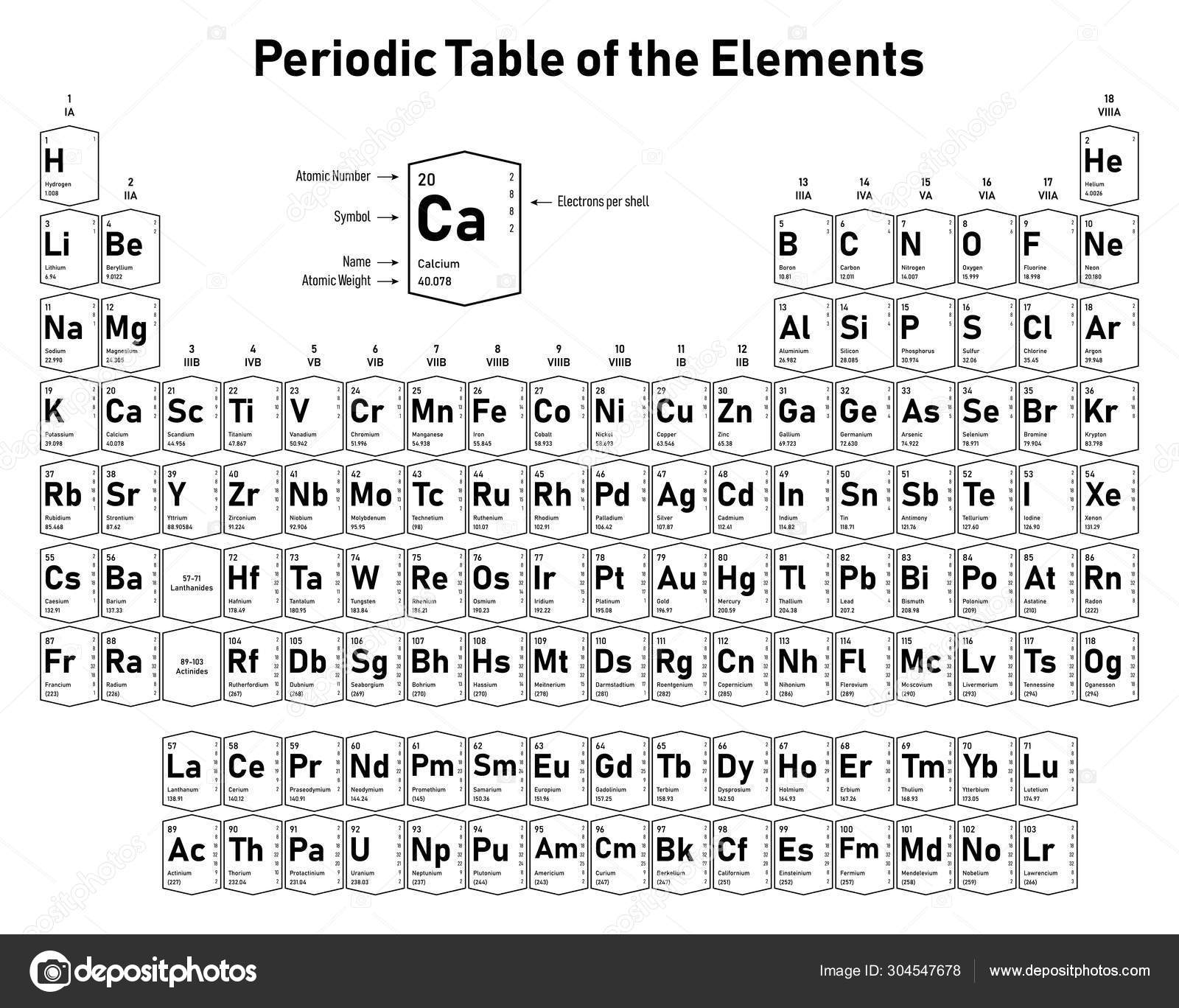
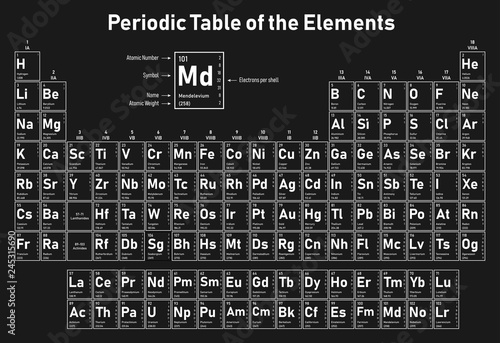
Periodic Table Electrons Per Shell
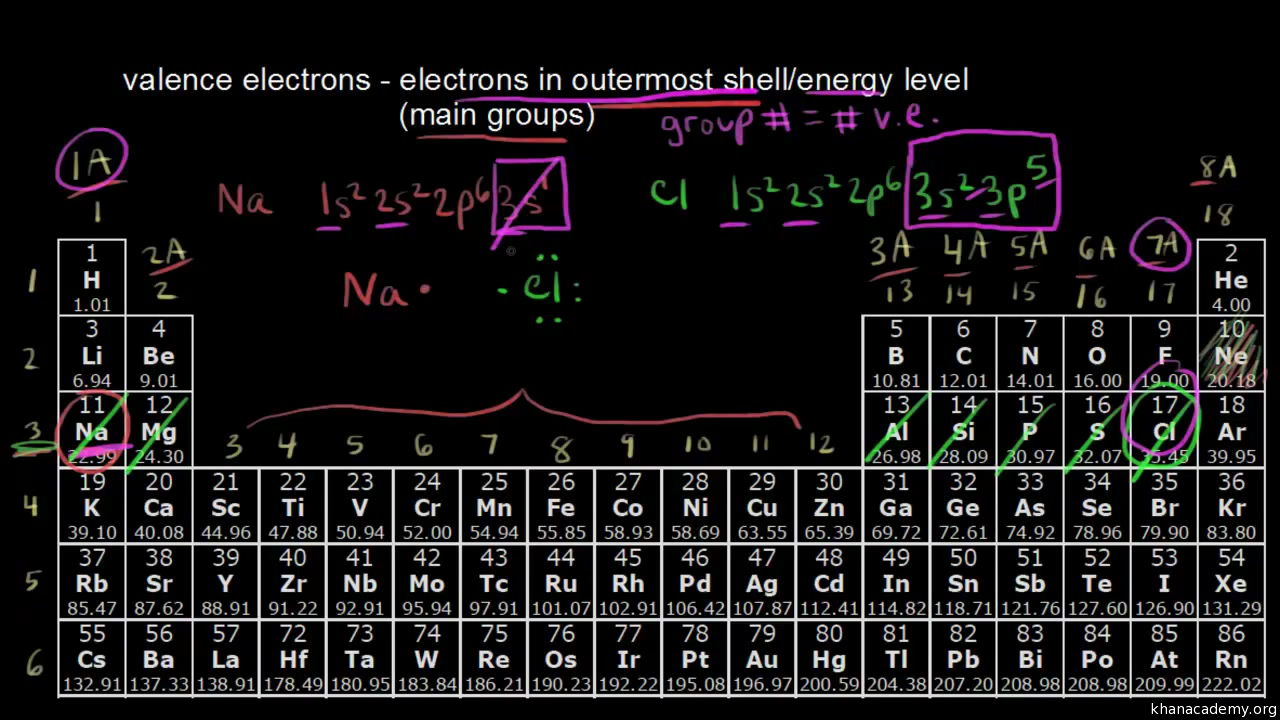
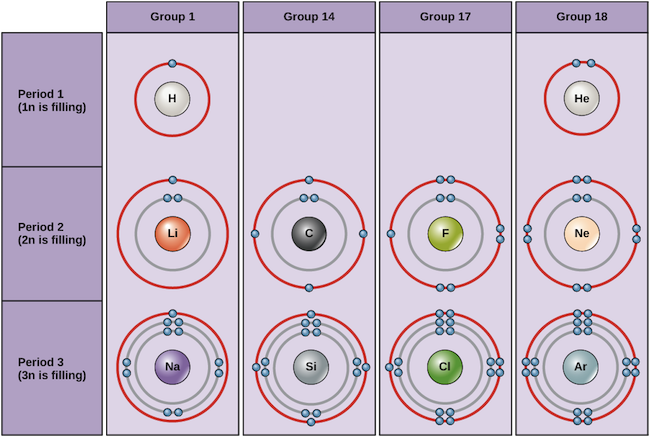
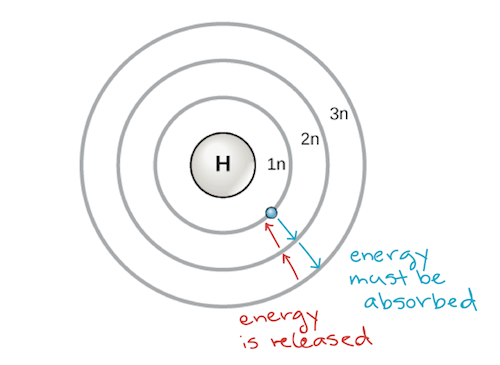
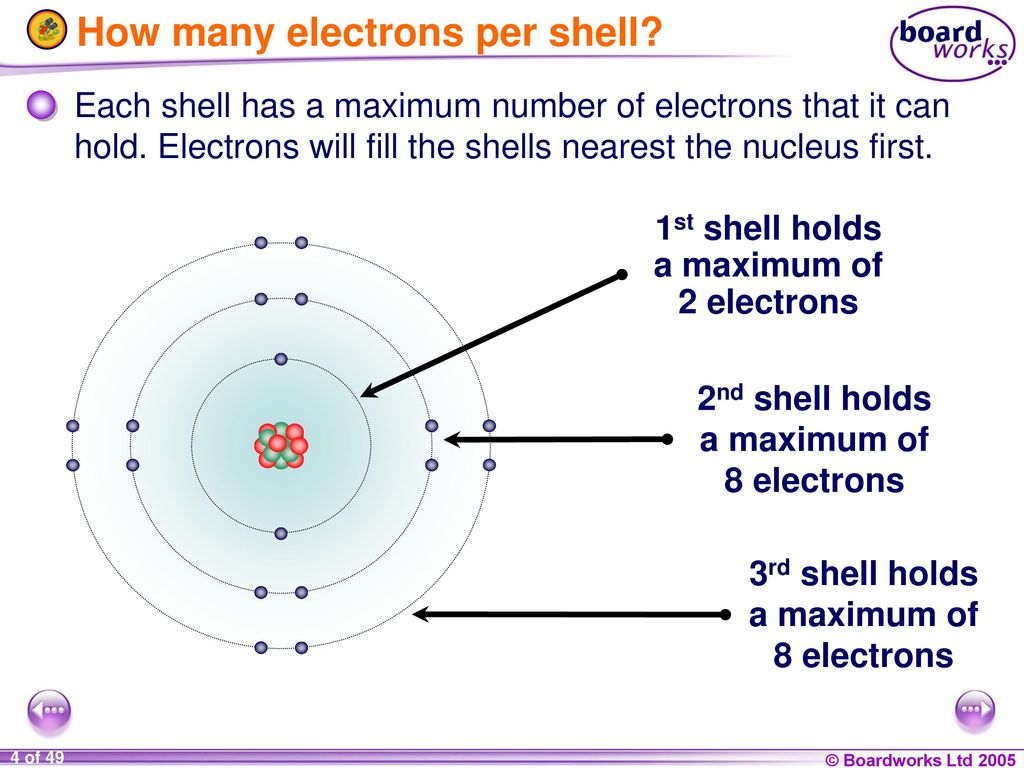
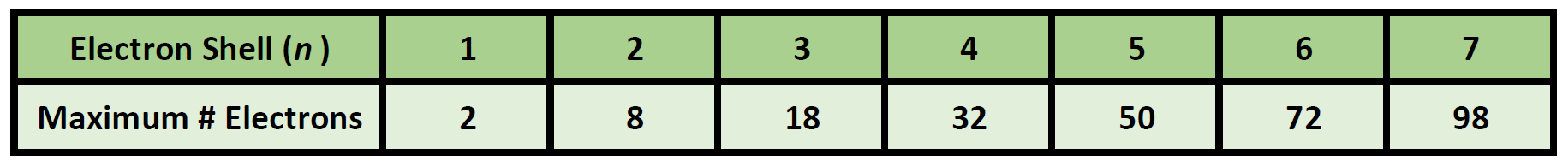
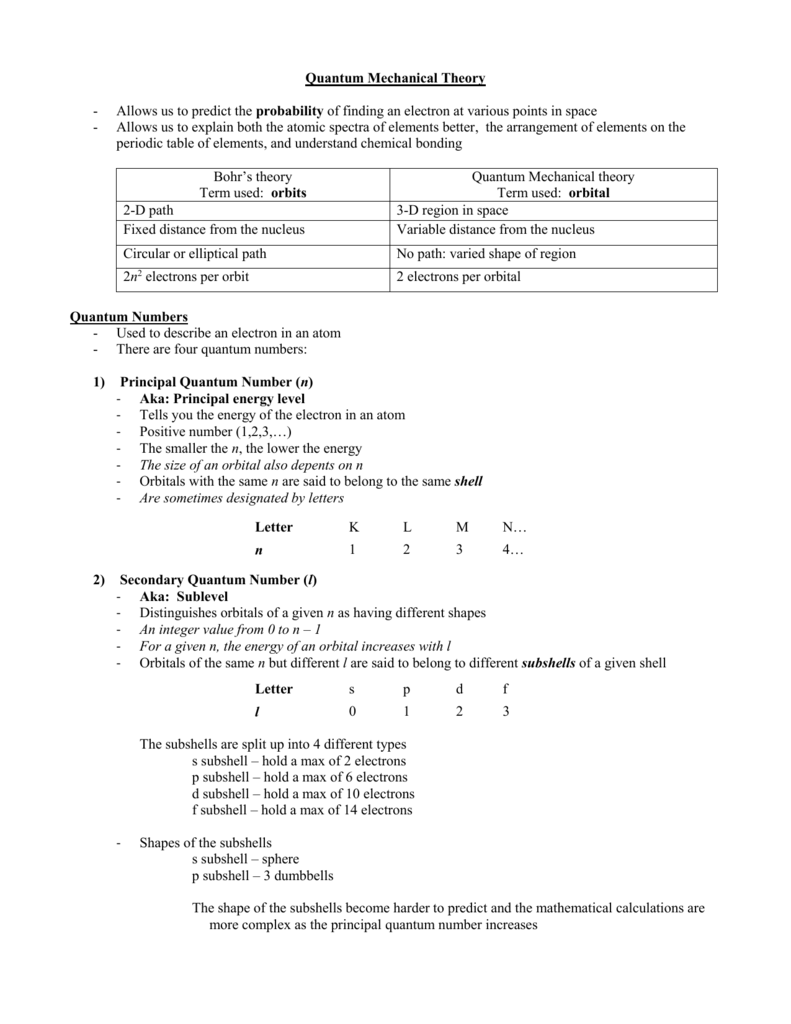
The general formula is that the nth shell can in principle hold up to 2n 2 electrons. The first shell can hold up to two electrons the second shell can hold up to eight 2 6 electrons the third shell can hold up to 18 2 6 10 and so on.
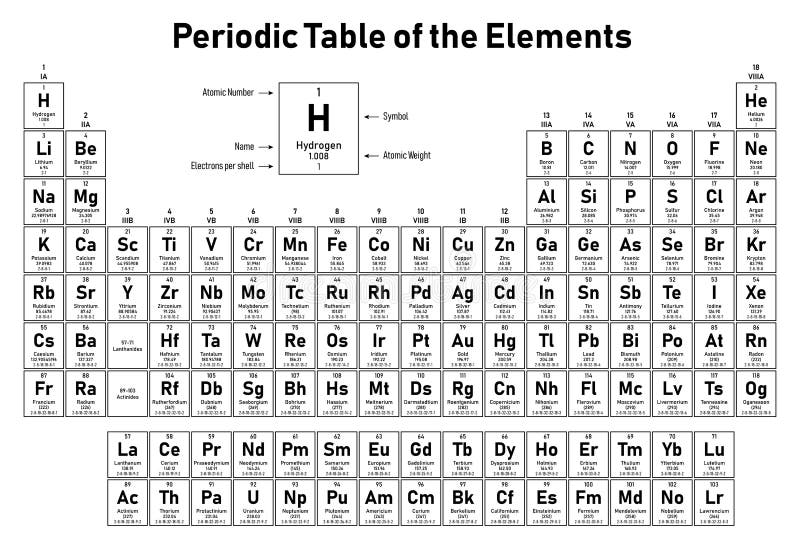
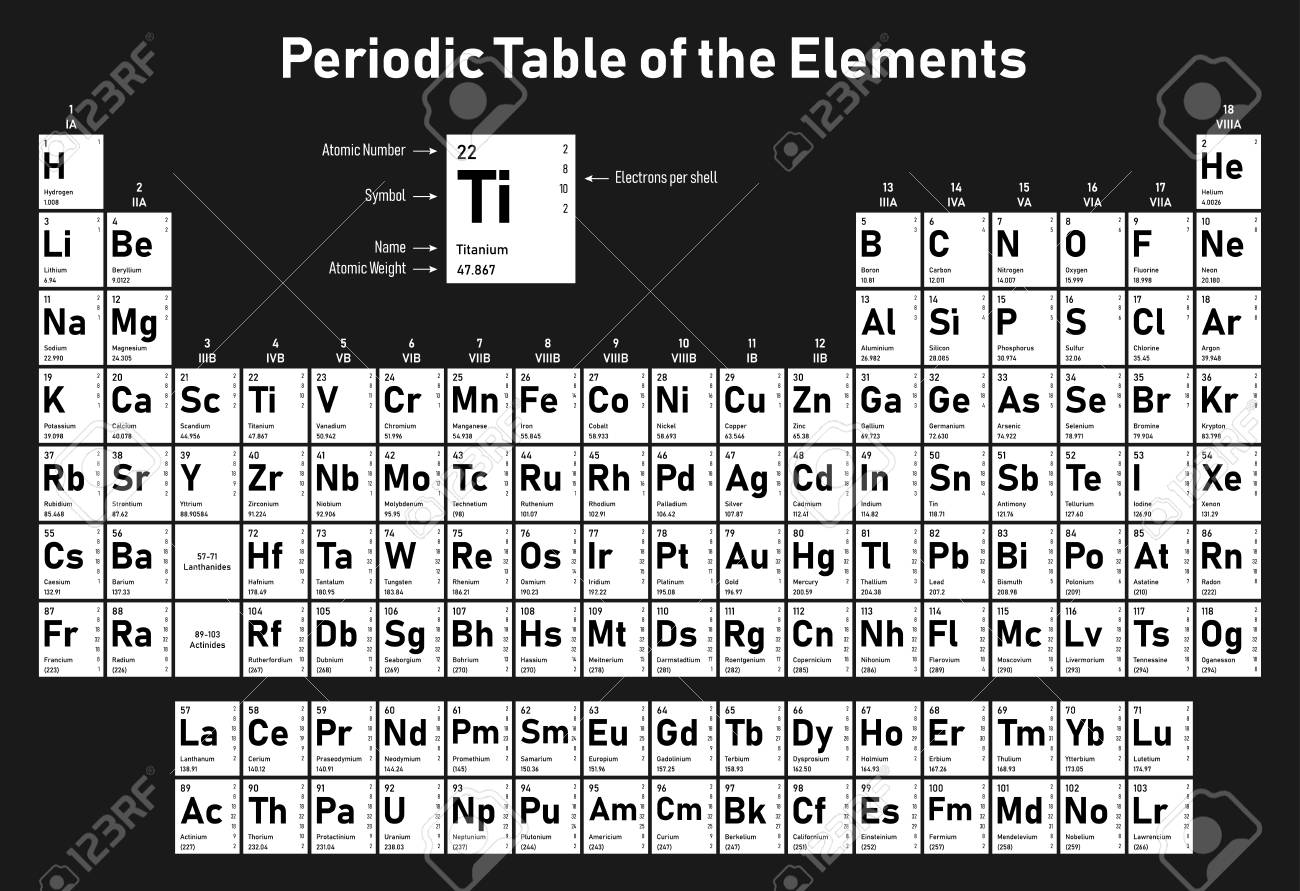
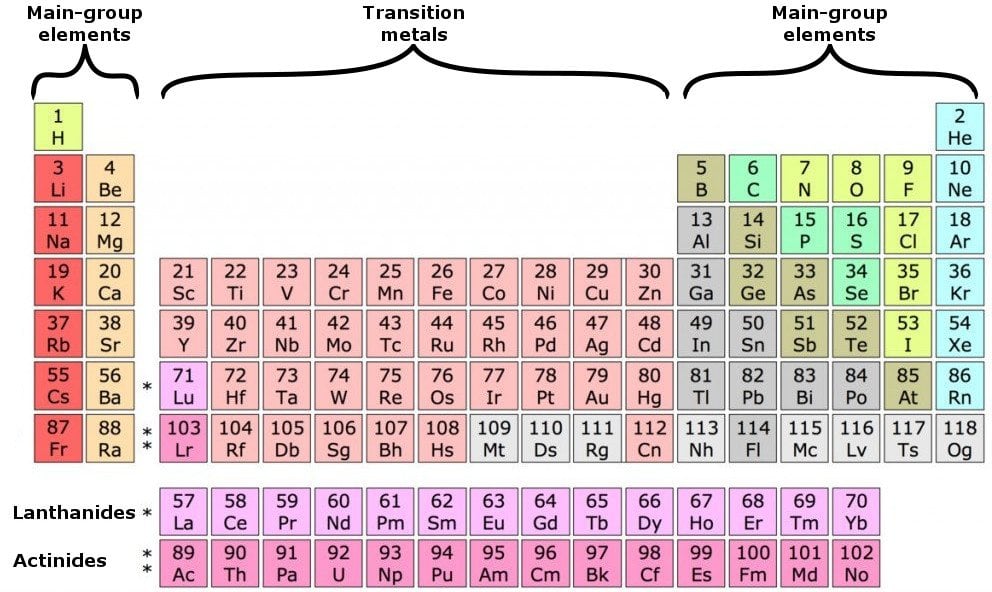
In the periodic table the elements are listed in order of increasing atomic number z.

Periodic table electrons per shell. I assume you are talking about group 18 of the table. Neils bohr gave the planetary model of an atom. The configuration of these electrons follows from the principles of quantum mechanics.
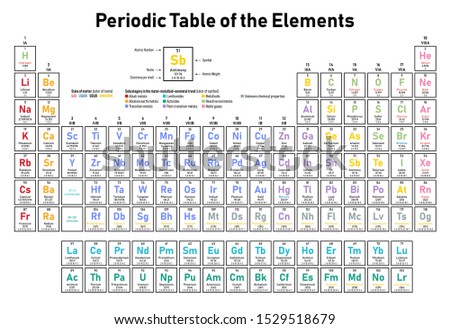
Full descriptions from write up sources. Each new period begins with one valence electron. Interactive periodic table with dynamic layouts showing names electrons oxidation trend visualization orbitals isotopes and compound search.
He was the first person to suggest the periodicity in the properties of the elements. Each shell can contain only a fixed number of electrons. The first two are located on the s sub shell with one electron on the p sub shell.
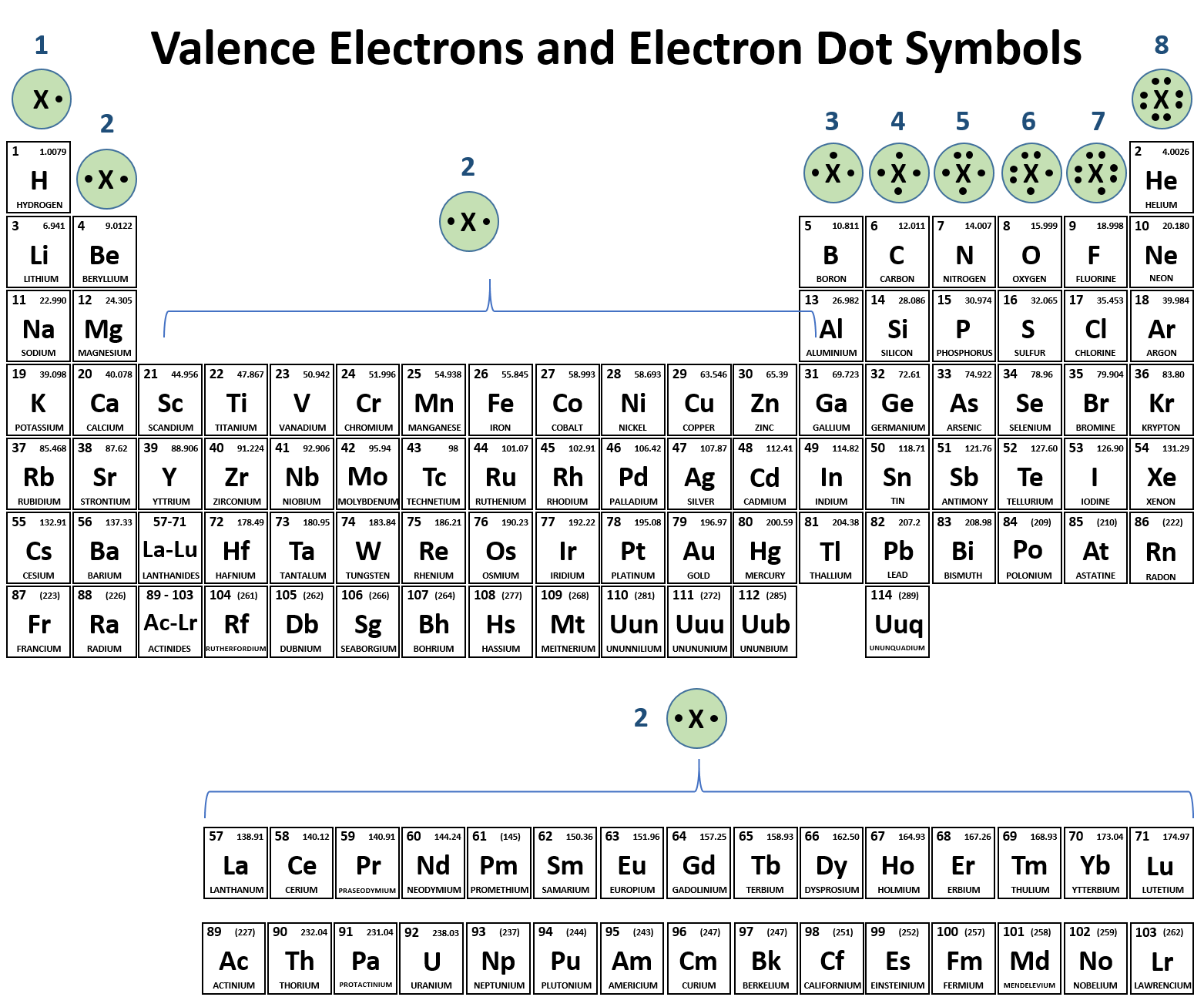
These elements have a full valence shell 8 electrons. The second electron shell has three electrons. The general formula is that the nth shell can in principle hold up to 2n 2 electrons.
The rule is as follows. Using an elements position in the periodic table to predict its properties electron configuration and reactivity. Group 18 of the periodic table of the elements are the noble gases.
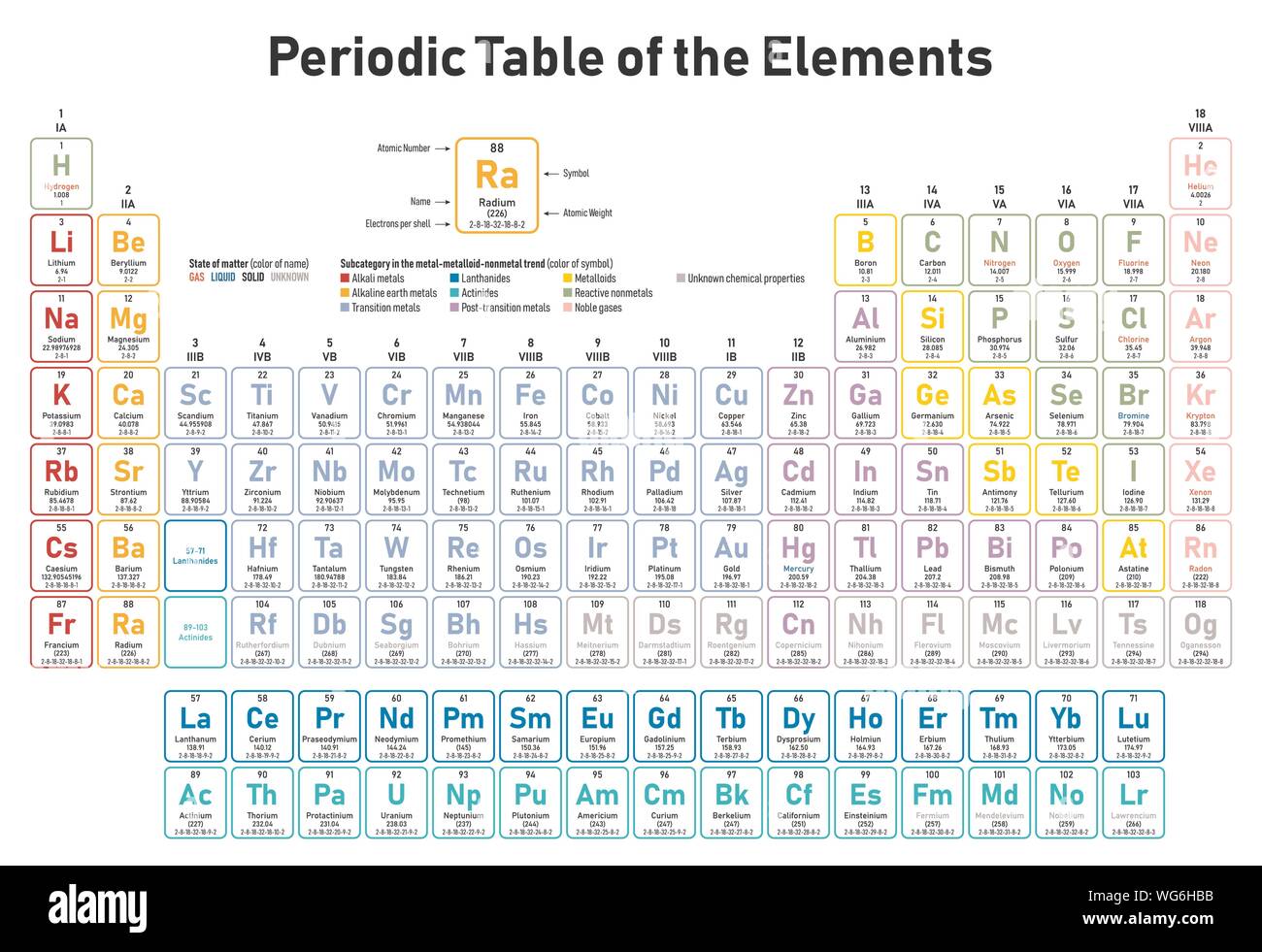
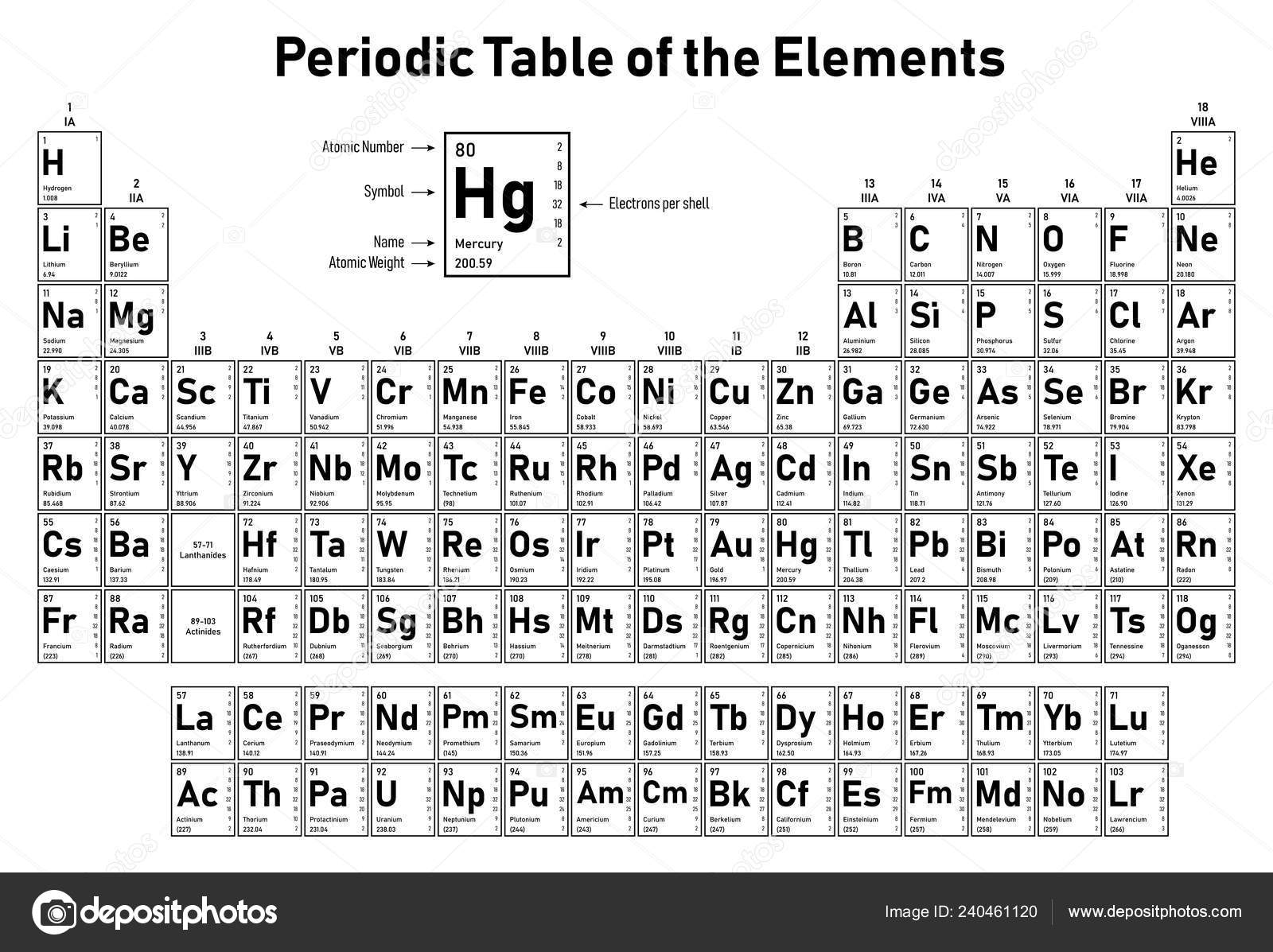
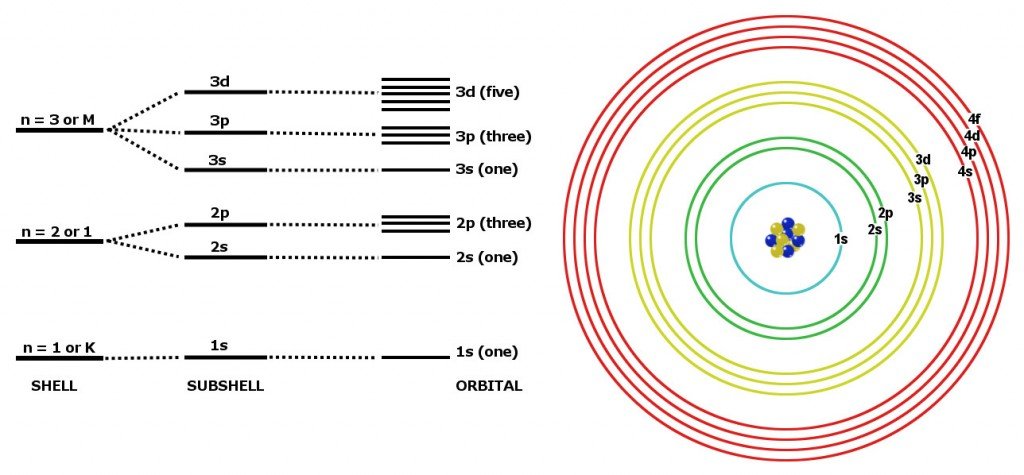
This notation indicates which electron shell first by a number the sub shell by the letter and how many electrons are present on the sub shell with a number. Exclude groups 3 through 12. He was the person to describe the arrangement of electrons electronic configuration in different orbitsshells.
Apply the rule of the periodic table to your element. Distribution of electrons in different orbits. Bohr atomic model forms the basis of the electronic structure of an atom.
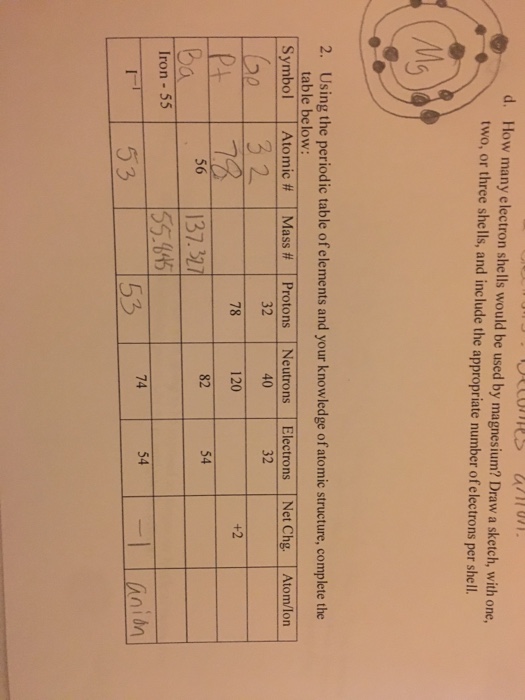
If an element is not a transition metal then valence electrons increase in number as you count groups left to right along a period. Each element is detailed with the name symbol and number of electrons in each shell. These are transitional metals which have special circumstances.
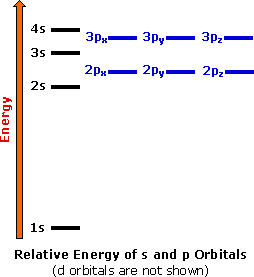
A common sub shell notation for boron is 1s2 2s2 2p1. If youre seeing this message it means were having trouble loading external resources on our website. The colour scheme is designed to match that used by wikipedia for its own element related articles.
The number of electrons in each elements electron shells particularly the outermost valence shell is the primary factor in determining its chemical bonding behavior. Each shell can contain only a fixed number of electrons. The colour scheme is designed to match that used by wikipedia for its own element related articles.
The first shell can hold up to two electrons the second shell can hold up to eight 2 6 electrons the third shell can hold up to 18 2 6 10 and so on.


/ecblocks-56a129535f9b58b7d0bc9f2e.jpg)













0 Response to "Periodic Table Electrons Per Shell"
Post a Comment