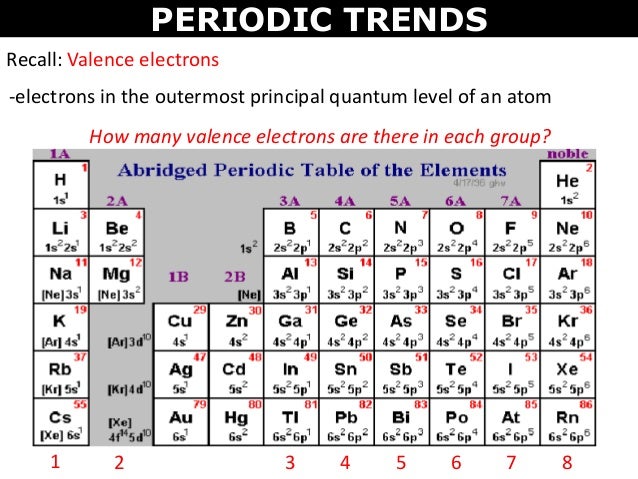
Valence Shell Electrons Periodic Table
When we study and observe the atom of an element we come across tiny subatomic particles called valence electrons. As expected that is exactly the number of electrons in its valence shell.
An electron cannot leave an atom easily when an electric field is applied and thus such an element can conduct only very small electric currents.
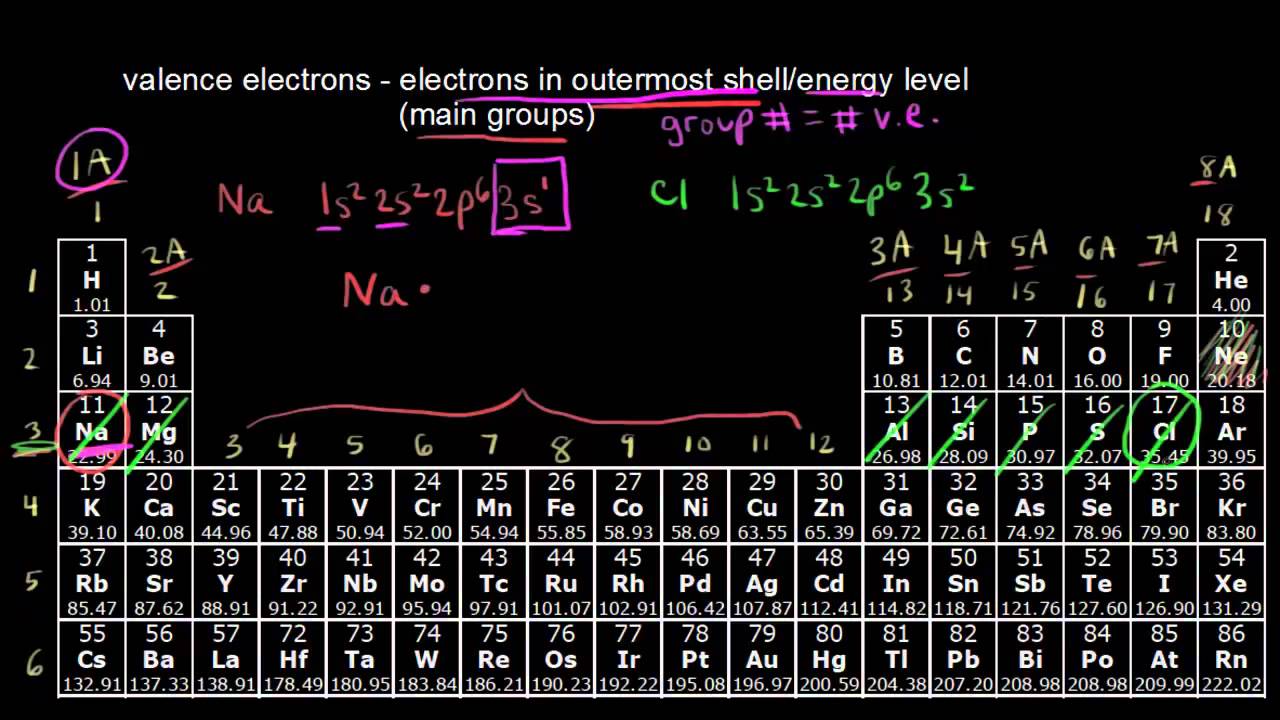
Valence shell electrons periodic table. Remember that an elements electron cloud will become more stable by filling emptying or half filling the shell. Valence electrons are the s and p electrons in the outermost shell. Lewis structures help us too track the valence electrons and predict the types of bond.
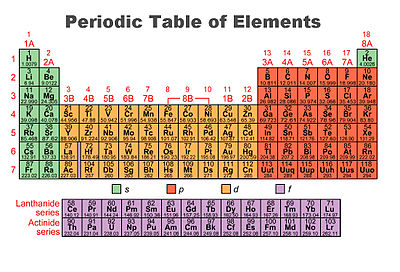
Periodic table of elements with valence electrons trends. For facts physical properties chemical properties structure and atomic properties of the specific element click on the element symbol in the below periodic table. Valence electrons are the electrons present in the outermost shell of an atom.
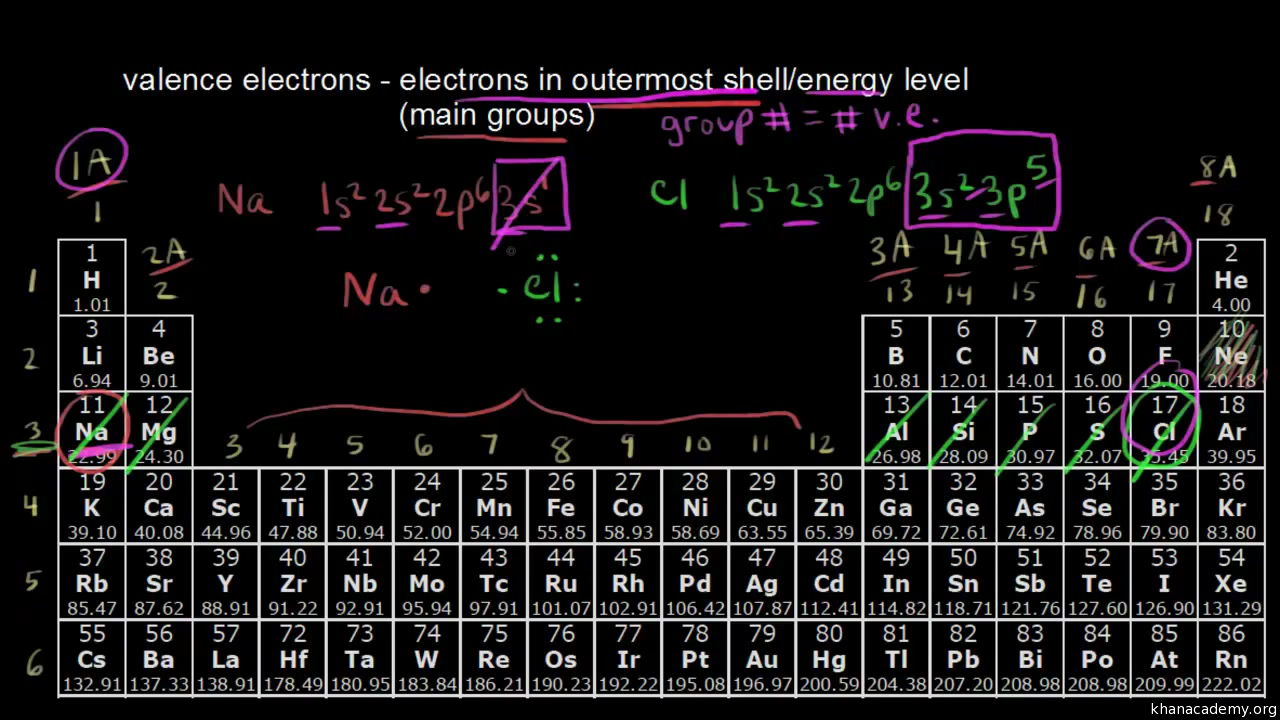
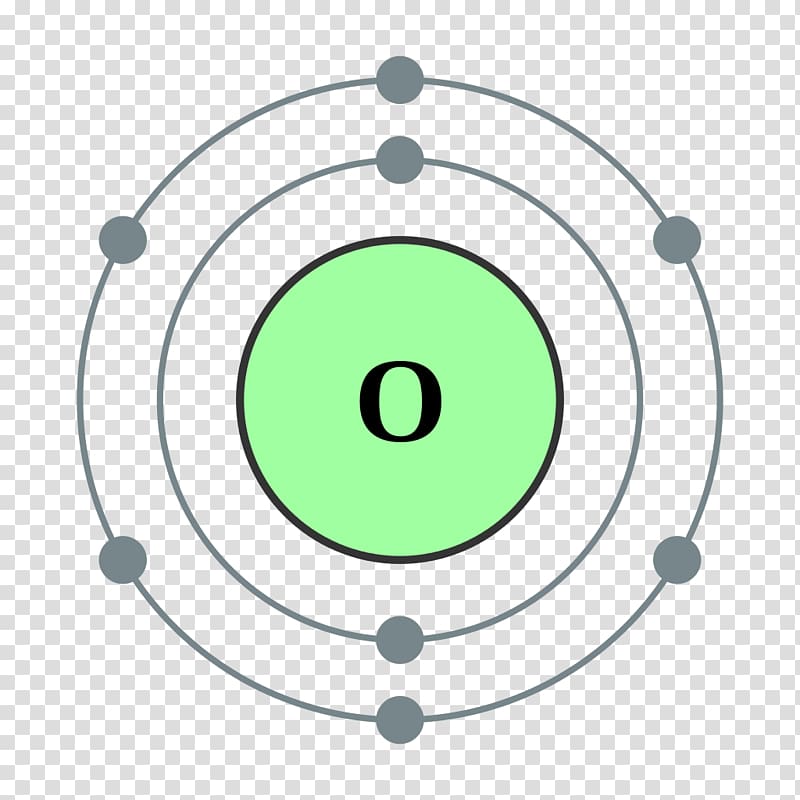
Accordingly in order to determine its valence electrons we must only seek the number in its ones place. It is called the valence shell. Accordingly valence electrons directly influence how elements behave in a chemical reaction.
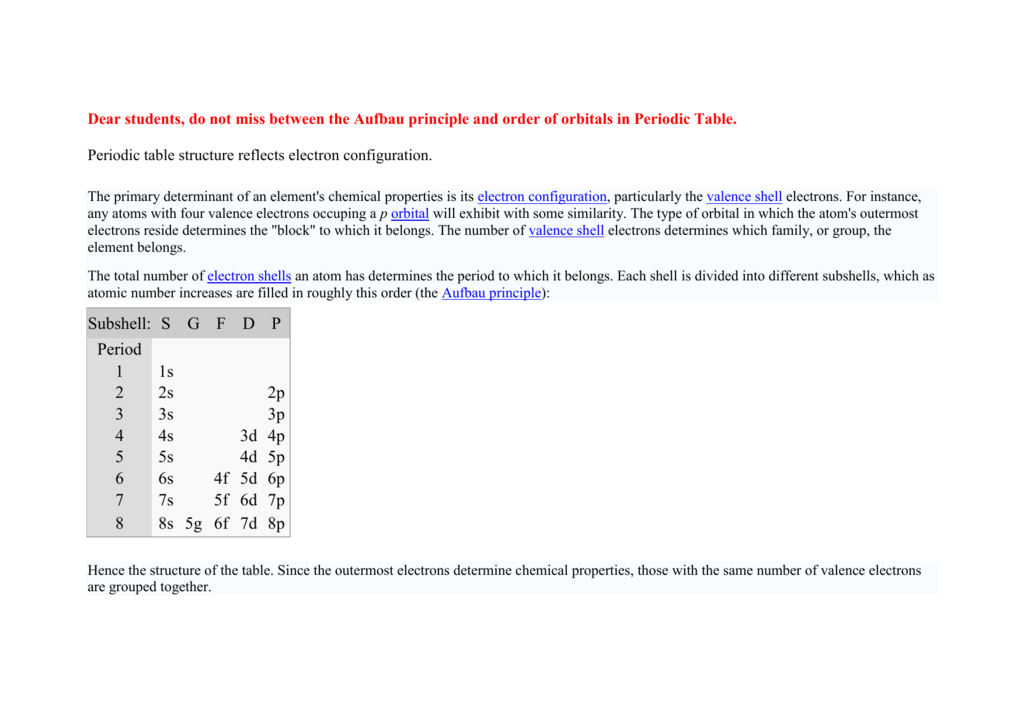
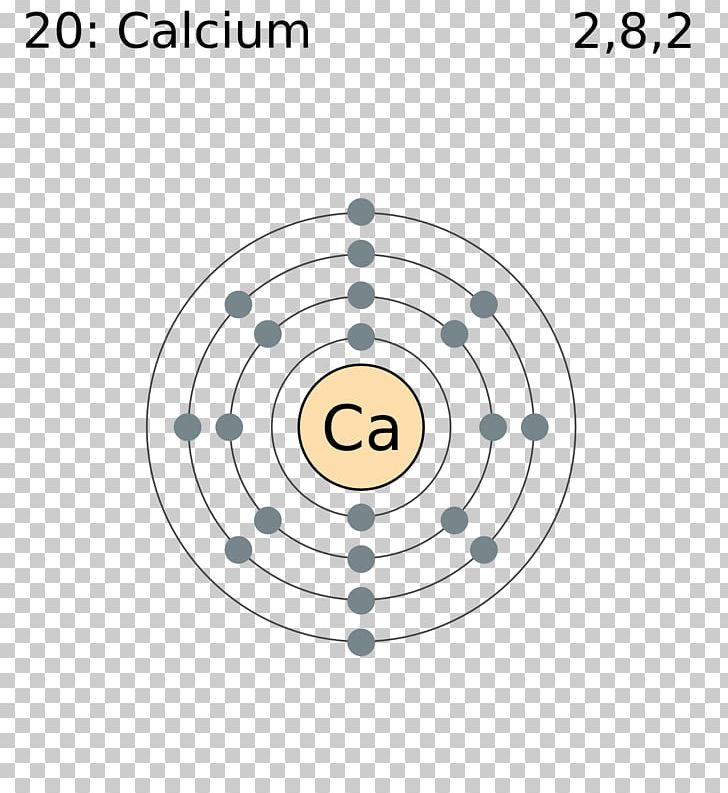
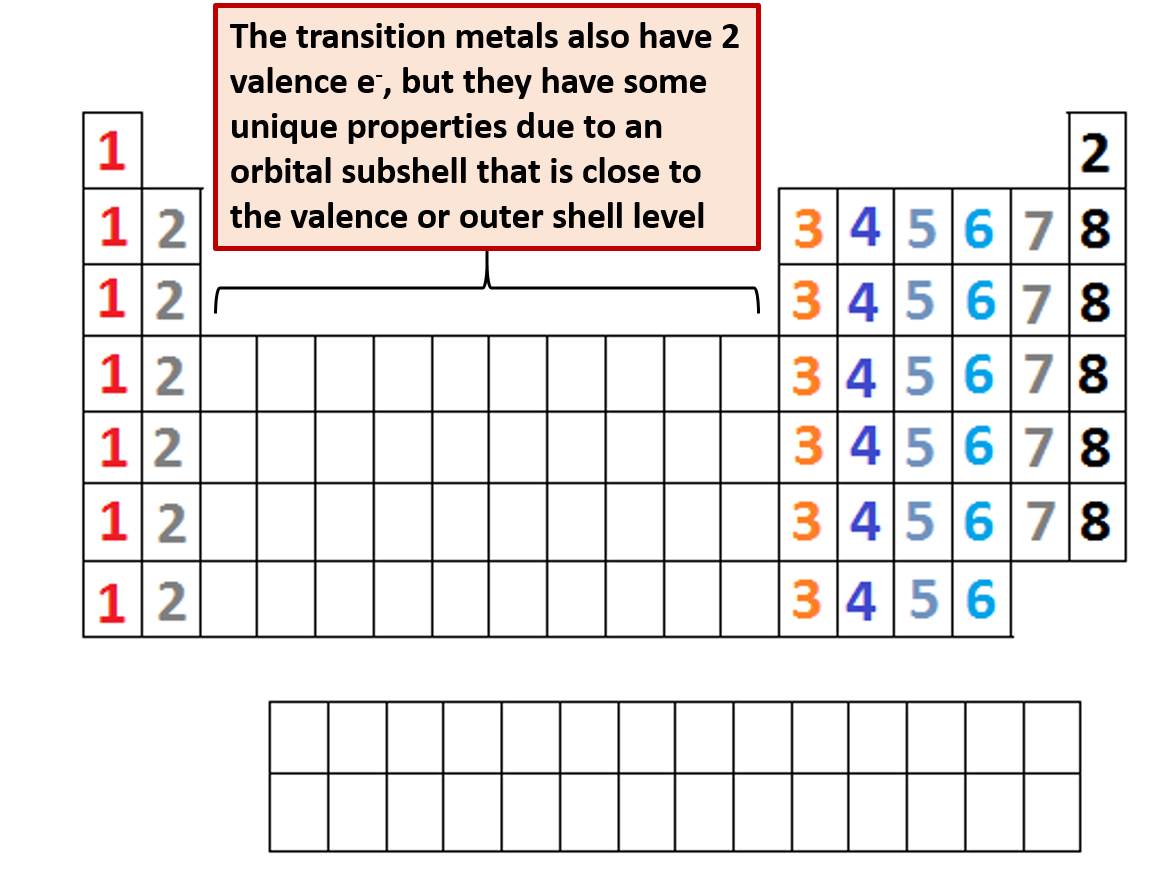
Also shells dont stack neatly one on top of another so dont always assume an elements valence is determined by the number of electrons in its outer shell. Each shell consists of one or more subshells and each subshell consists of one or more atomic orbitals. By definition valence electrons travel in the subshell farthest away from the nucleus of the atom.
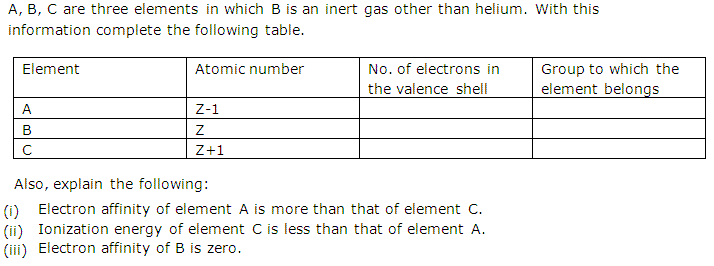
Here is a table of element valences. The electrons present in the inner shell are core electrons. The electrons in the outermost occupied shell or shells determine the chemical properties of the atom.
If youre seeing this message it means were having trouble loading external resources on our website. Its ionization energy is large. Such an element is found toward the right of the periodic table and it has a valence shell that is at least half full the exception is boron.
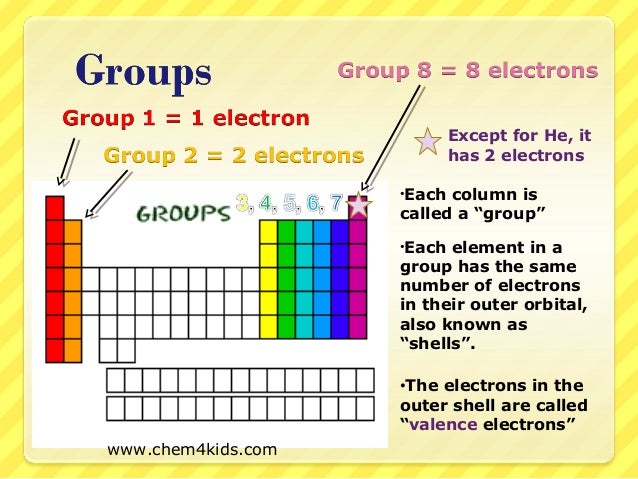
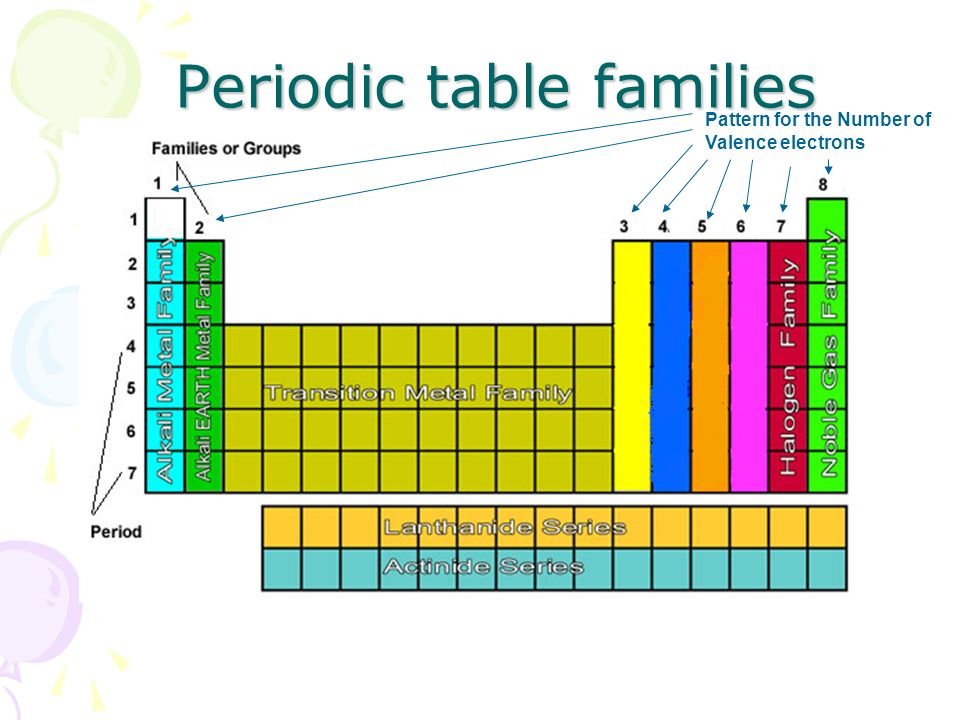
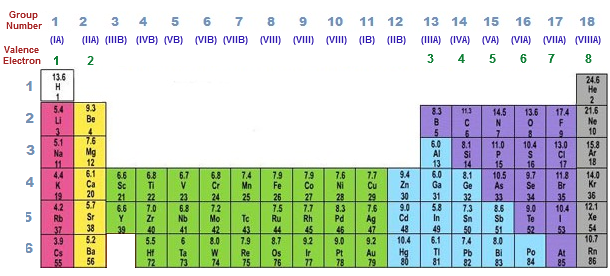
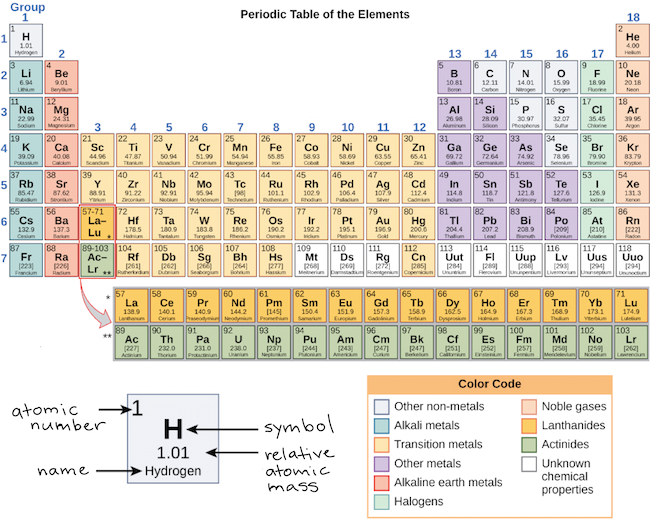
Now locate the element that you want to find the valence electrons for on the table. Using an elements position in the periodic table to predict its properties electron configuration and reactivity. In the below periodic table you can see the trend of valence electrons.
Or you can consider chlorine in group 17. You can do this with its chemical symbol the letters in each box its atomic number the number in the top left of each box or any of the other pieces of information available to you on the table. Each electron shell is composed of one or more subshells.
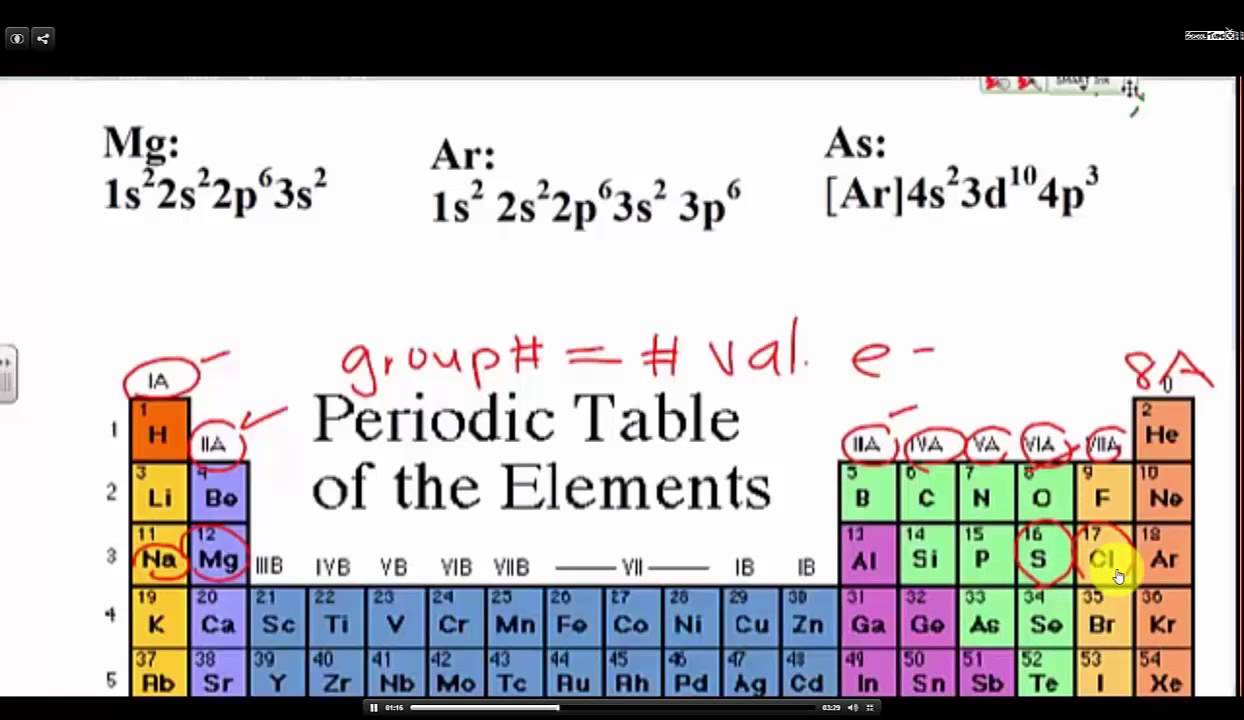
You can easily determine the number of valence electrons an atom can have by looking at its group in the periodic table. For example atoms in groups 1 and 2 have 1 and 2 valence electrons respectively. Atoms tend to accept or lose electrons if doing so will result in a full outer shell.























0 Response to "Valence Shell Electrons Periodic Table"
Post a Comment